Expanding the Allelic and Clinical Heterogeneity of Movement Disorders Linked to Defects of Mitochondrial Adenosine Triphosphate Synthase
- PMID: 40276935
- PMCID: PMC12273622
- DOI: 10.1002/mds.30209
Expanding the Allelic and Clinical Heterogeneity of Movement Disorders Linked to Defects of Mitochondrial Adenosine Triphosphate Synthase
Abstract
Background: Defects of mitochondrial ATP synthase (ATPase) represent an emerging, yet incompletely understood group of neurodevelopmental diseases with abnormal movements.
Objective: The aim of this study was to redefine the phenotypic and mutational spectrum of movement disorders linked to the ATPase subunit-encoding genes ATP5F1A and ATP5F1B.
Methods: We recruited regionally distant patients who had been genome or exome sequenced. Fibroblast cultures from two patients were established to perform RNA sequencing, immunoblotting, mass spectrometry-based high-throughput quantitative proteomics, and ATPase activity assays. In silico three-dimensional missense variant modeling was performed.
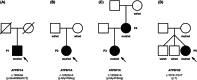
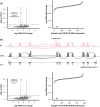
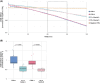
Results: We identified a patient with developmental delay, myoclonic dystonia, and spasticity who carried a heterozygous frameshift c.1404del (p.Glu469Serfs*3) variant in ATP5F1A. The patient's cells exhibited significant reductions in ATP5F1A mRNA, underexpression of the α-subunit of ATPase in association with other aberrantly expressed ATPase components, and compromised ATPase activity. In addition, a novel deleterious heterozygous ATP5F1A missense c.1252G>A (p.Gly418Arg) variant was discovered, shared by three patients from two families with hereditary spastic paraplegia (HSP). This variant mapped to a functionally important intersubunit communication site. A third heterozygous variant, c.1074+1G>T, affected a canonical donor splice site of ATP5F1B and resulted in exon skipping with significantly diminished ATP5F1B mRNA levels, as well as impaired ATPase activity. The associated phenotype consisted of cerebral palsy (CP) with prominent generalized dystonia.
Conclusions: Our data confirm and expand the role of dominant ATP5F1A and ATP5F1B variants in neurodevelopmental movement disorders. ATP5F1A/ATP5F1B-related ATPase diseases should be considered as a cause of dystonia, HSP, and CP. © 2025 The Author(s). Movement Disorders published by Wiley Periodicals LLC on behalf of International Parkinson and Movement Disorder Society.
Keywords: ATP synthase; ATP5F1A; ATP5F1B; cerebral palsy; dominant variant; dystonia; mitochondrial disease; spasticity.
© 2025 The Author(s). Movement Disorders published by Wiley Periodicals LLC on behalf of International Parkinson and Movement Disorder Society.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

