Rozanolixizumab for Myasthenia Gravis: a breakthrough treatment and future prospects
- PMID: 40277145
- PMCID: PMC12045563
- DOI: 10.1080/1750743X.2025.2491295
Rozanolixizumab for Myasthenia Gravis: a breakthrough treatment and future prospects
Abstract
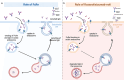
Myasthenia gravis is a rare chronic autoimmune disorder affecting the post-synaptic neuromuscular junction, primarily mediated by pathogenic immunoglobulin G (IgG) targeting specific proteins like acetylcholine receptor (AChR), muscle-specific tyrosine kinase (MuSK), and low-density lipoprotein receptor-related protein 4 (LRP4). Modulating pathogenic IgG is a promising approach for disease management. Rozanolixizumab, a human IgG4 neonatal Fc receptor (FcRn) inhibitor, enhances the degradation of pathogenic IgG by 78%, marking a significant advancement in treating generalized myasthenia gravis. It offers effective management for patients with AChR or MuSK antibodies and is administered subcutaneously with mild to moderate adverse events. However, the safety and efficacy of rozanolixizumab require further validation through real-world post-marketing studies. If current trial results are confirmed, rozanolixizumab may become a preferred treatment option for myasthenia gravis in the near future. This review examines clinical trials evaluating the pharmacokinetics, efficacy, and safety of rozanolixizumab in patients with generalized myasthenia gravis and discusses ongoing trials and future research directions.
Keywords: AChR; FcRn inhibitor; MuSK; generalized myasthenia gravis; immunotherapy; rozanolixizumab.
Plain language summary
Myasthenia gravis is a rare disease where the immune system attacks nerve-muscle connections, causing muscle weakness. This happens because the body produces harmful antibodies against certain proteins involved in muscle movement. Rozanolixizumab is a new treatment that targets these harmful antibodies. It works by helping to remove these antibodies from the body more effectively. This treatment has shown promise in helping people with myasthenia gravis, especially those with specific types of antibodies. It is given as an injection under the skin and usually has only mild to moderate side effects. However, more studies are needed to confirm how well it works and how safe it is in everyday use. If future research supports the current findings, rozanolixizumab might become a top choice for treating this condition. This review looks at the studies done so far on rozanolixizumab, including how the drug behaves in the body, how effective it is, and its safety. It also covers ongoing research and what the future might hold for this treatment.
Conflict of interest statement
V Bril declares consultancy for Grifols, CSL, UCB, Argenx, Takeda, Alnylam, Octapharma, Pfizer, Powell Mansfield Inc, Akcea, Ionis, Immunovant, Sanofi, Momenta (J&J), Roche, Janssen, AZ-Alexion, NovoNordisk andJapan Tobacco; and research support from AZ-Alexion, Grifols, CSL, UCB, Argenx, Takeda, Octapharma, Akcea, Momenta (J&J) and Immunovant, Ionis. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Figures
Similar articles
-
Rozanolixizumab in generalized myasthenia gravis: Pooled analysis of the Phase 3 MycarinG study and two open-label extensions.J Neuromuscul Dis. 2025 Mar;12(2):218-230. doi: 10.1177/22143602241305511. Epub 2025 Mar 4. J Neuromuscul Dis. 2025. PMID: 40033991 Clinical Trial.
-
Efficacy and safety of rozanolixizumab in patients with muscle-specific tyrosine kinase autoantibody-positive generalised myasthenia gravis: a subgroup analysis of the randomised, double-blind, placebo-controlled, adaptive phase III MycarinG study.Ther Adv Neurol Disord. 2024 Sep 12;17:17562864241273036. doi: 10.1177/17562864241273036. eCollection 2024. Ther Adv Neurol Disord. 2024. PMID: 39297052 Free PMC article.
-
An evaluation of rozanolixizumab-noli for the treatment of anti-AChR and anti-MuSK antibody-positive generalized myasthenia gravis.Expert Opin Biol Ther. 2023 Jul-Dec;23(12):1163-1171. doi: 10.1080/14712598.2023.2296126. Epub 2023 Dec 28. Expert Opin Biol Ther. 2023. PMID: 38099334 Review.
-
Long-term safety of cyclical rozanolixizumab in patients with generalized myasthenia gravis: Results from the Phase 3 MycarinG study and an open-label extension.J Neuromuscul Dis. 2025 Mar;12(2):231-243. doi: 10.1177/22143602241308181. Epub 2025 Mar 4. J Neuromuscul Dis. 2025. PMID: 40034001 Clinical Trial.
-
Therapies Directed Against B-Cells and Downstream Effectors in Generalized Autoimmune Myasthenia Gravis: Current Status.Drugs. 2019 Mar;79(4):353-364. doi: 10.1007/s40265-019-1065-0. Drugs. 2019. PMID: 30762205 Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
