Safety and Efficacy of a Selective Inhibitor of Cyclin-dependent Kinase 9 (KB-0742) in Patients with Recurrent or Metastatic Adenoid Cystic Carcinoma
- PMID: 40277179
- PMCID: PMC12056915
- DOI: 10.1158/2767-9764.CRC-25-0015
Safety and Efficacy of a Selective Inhibitor of Cyclin-dependent Kinase 9 (KB-0742) in Patients with Recurrent or Metastatic Adenoid Cystic Carcinoma
Abstract
Purpose: Adenoid cystic carcinoma (ACC) is a rare salivary gland malignancy of the head and neck. Recurrent or metastatic ACC has very limited therapeutic options. Cyclin-dependent kinase 9 (CDK9) is a key factor in the oncogenic transcriptional regulatory network, and inhibition of CDK9 may prove beneficial in MYC-dependent tumors such as ACC.
Patients and methods: A first-in-human, phase I, two-part dose-escalation and -expansion clinical trial (NCT04718675) enrolled patients with advanced solid tumors reliant on transcription factor activation to receive KB-0742, an oral selective inhibitor of CDK9. The primary endpoint was to establish safety/tolerability while nominating a recommended phase II dose (RP2D). Secondary endpoints included characterization of pharmacokinetics and assessment of preliminary efficacy.
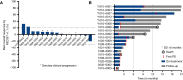
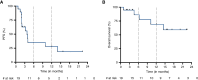
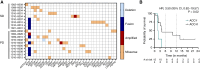
Results: Among 19 patients with ACC enrolled in dose expansion at the RP2D (60 mg orally 3 days on and 4 days off each week during a 28-day cycle), the regimen was well tolerated with mild gastrointestinal toxicity and fatigue; a single grade 3 treatment-related adverse event was observed (elevated γ-glutamyl transferase). One patient discontinued for gastrointestinal toxicity. Although no responses were observed, nine of 16 (56%) eligible patients had stable disease, with four experiencing >6 months of stability. Six-month progression-free survival was 37% (95% confidence interval, 14.2-59.8). Most patients had the more indolent type II ACC phenotype and 10 (53%) had MYB alterations.
Conclusions: This dose-expansion cohort exploring the novel CDK9 inhibitor KB-0742 in patients with advanced ACC established favorable tolerability at the RP2D. Disease stabilization was observed in some patients despite a limited efficacy signal.
Significance: A first-in-human, phase I trial explored the safety and preliminary efficacy of the CDK9 inhibitor KB-0742 in patients with advanced, transcription factor-dependent solid tumors including ACC. KB-0742 was well tolerated with evidence of disease stabilization observed among some patients with ACC, but overall therapeutic efficacy was limited.
©2025 The Authors; Published by the American Association for Cancer Research.
Conflict of interest statement
G.J. Hanna reports grants from Kronos Bio during the conduct of the study, as well as grants from Adenoid Cystic Carcinoma Research Foundation, Actuate, Cellestia, Gateway for Cancer Research, Genentech, and PHASE ONE Foundation and grants and personal fees from Elevar, Kura Oncology, Remix, and Rgenta outside the submitted work. G.M. Cote reports other support from Gilead Sciences, PharmaMar, Servier, MacroGenics, Eisai, Merck KGaA/EMD, Repare, Foghorn, Jazz Pharmaceuticals, RAIN, BioAtla, Inhibrx, Ikena, C4 Therapeutics, Kronos Bio, Bavarian Nordic, and Pyxis and personal fees from Chordoma Foundation and Ikena outside the submitted work. R. Chugh reports grants from Kronos Bio during the conduct of the study, as well as personal fees from Deciphera Pharmeceuticals and Recordati Rare Diseases, grants and personal fees from SpringWorks Therapeutics and Inhibrx, and grants from Ayala Pharmaceuticals, Astex Pharmaceuticals, Cogent Biosciences, Pfizer, PharmaMar, Polaris, and GlaxoSmithKline outside the submitted work. J.S. Thomas reports personal fees from Kura Oncology during the conduct of the study, as well as personal fees from Coherus BioSciences outside the submitted work. J. Malhotra reports other support from Kronos Bio during the conduct of the study. T. Hood reports grants from Kronos Bio during the conduct of the study; other support from Kronos Bio outside the submitted work; a patent to Patent pending; and employment with Kronos Bio during the conduct of this study. L.A. Carvajal reports other support from Kronos Bio during the conduct of the study, as well as other support from Kronos Bio outside the submitted work. M.A. Villalona-Calero reports personal fees from Kronos Bio, grants from Kronos Bio, and other support from City of Hope National Medical Center during the conduct of the study, as well as personal fees from Eli Lilly and Company, grants from NCI, Amgen, Cytoimmune, Nectin Therapeutics, Cyclacel Pharmaceuticals, and IDEAYA Biosciences, and other support from University of California Irvine outside the submitted work. No disclosures were reported by the other authors.
Figures
References
-
- Coca-Pelaz A, Rodrigo JP, Bradley PJ, Vander Poorten V, Triantafyllou A, Hunt JL, et al. . Adenoid cystic carcinoma of the head and neck–An update. Oral Oncol 2015;51:652–61. - PubMed
-
- Hanna GJ, Bae JE, Lorch JH, Schoenfeld JD, Margalit DN, Tishler RB, et al. . Long-term outcomes and clinicogenomic correlates in recurrent, metastatic adenoid cystic carcinoma. Oral Oncol 2020;106:104690. - PubMed
-
- Kacew AJ, Hanna GJ. Systemic and targeted therapies in adenoid cystic carcinoma. Curr Treat Options Oncol 2023;24:45–60. - PubMed
-
- Licitra L, Cavina R, Grandi C, Palma SD, Guzzo M, Demicheli R, et al. . Cisplatin, doxorubicin and cyclophosphamide in advanced salivary gland carcinoma. A phase II trial of 22 patients. Ann Oncol 1996;7:640–2. - PubMed
-
- Laurie SA, Siu LL, Winquist E, Maksymiuk A, Harnett EL, Walsh W, et al. . A phase 2 study of platinum and gemcitabine in patients with advanced salivary gland cancer: a trial of the NCIC Clinical Trials Group. Cancer 2010;116:362–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

