Mer overexpression in Methanosarcina acetivorans affects growth and methanogenesis during substrate adaptation
- PMID: 40277366
- PMCID: PMC12093972
- DOI: 10.1128/aem.00675-25
Mer overexpression in Methanosarcina acetivorans affects growth and methanogenesis during substrate adaptation
Abstract
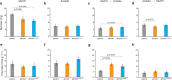
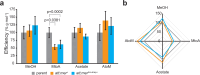
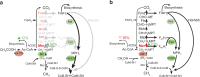
Evidence suggests that multienzyme complexes are involved in biological methane production (methanogenesis), although the composition of the Wolfe Cycle methanogenesis complexes may vary between diverse methanoarchaeal taxa. Methylenetetrahydromethanopterin reductase (Mer) is the first committed step in C1 oxidation to CO2 during methylotrophic methanogenesis. However, Mer is downregulated when cells use acetate as a substrate. We hypothesized that Mer overexpression during methylotrophic methanogenesis would be beneficial, while overexpression during acetoclastic methanogenesis would be detrimental for energy conservation. To test this hypothesis, we overexpressed Mer and characterized strain physiology on methanol, acetate, and when switching substrates. We found that Mer overexpression results in faster growth on methanol, with less C fixation into biomass, and no effect on methanogenesis. Growth on acetate was not affected by Mer overexpression, but switching between substrates was affected. The native Mer overexpressing strain was slower to adjust from methanol to acetate and vice-versa. These data suggest that tight regulation of Mer expression is necessary to regulate C flux through methylotrophic versus acetoclastic methanogenesis pathways in Methanosarcina.IMPORTANCEMethanoarchaea thrive near the "thermodynamic limit of life" and have likely evolved efficient mechanisms to control flux of substrates to conserve energy. Methylenetetrahydromethanopterin reductase (Mer) is a highly conserved, key enzyme in the Wood-Ljungdahl and Wolfe Cycle methanogenesis pathways. Our study sheds light on how Mer enzyme stoichiometry affects methanogenesis and suggests avenues for engineering the organism to promote renewable fuel or bioproduct synthesis.
Keywords: Methanosarcina; Wood-Ljungdahl; archaea; methane; methanogenesis; one-carbon.
Conflict of interest statement
N.R.B. has disclosed a significant financial interest in RollingCircle Biotech, LLC, and Molecular Trait Evolution, LLC. All other authors declare none.
Figures


References
-
- Shen Y, Linville JL, Urgun-Demirtas M, Mintz MM, Snyder SW. 2015. An overview of biogas production and utilization at full-scale wastewater treatment plants (WWTPs) in the United States: challenges and opportunities towards energy-neutral WWTPs. Renew Sustain Energy Rev 50:346–362. doi: 10.1016/j.rser.2015.04.129 - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

