Modern Emerging Biosensing Methodologies for the Early Diagnosis and Screening of Ovarian Cancer
- PMID: 40277517
- PMCID: PMC12024575
- DOI: 10.3390/bios15040203
Modern Emerging Biosensing Methodologies for the Early Diagnosis and Screening of Ovarian Cancer
Abstract
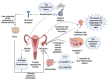
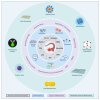
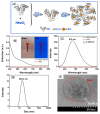
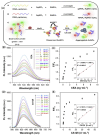
Ovarian cancer (OC) is one of the leading causes of gynecological cancer-related death worldwide. Late diagnosis at advanced stages of OC is the reason for a higher mortality rate. Earlier diagnosis and proper treatment are important for improving the prognosis of OC patients. Biosensors offer accurate, low-cost, rapid, and user-friendly devices that can be employed for the detection of OC-specific biomarkers in the early stage. Therefore, it is important to consider the potential biomarkers in the biological fluids to confirm the OC prognosis. Out of many biomarkers, the most commonly tested clinically is cancer antigen 125 (CA-125). However, CA-125 is considered to be a poor biomarker for OC diagnosis. Several biosensing methods were developed for the sensitive and quantitative detection of each biomarker. In abnormal expression in OC patients, nucleic acids, enzymes, cells, and exosomes are used as target biomarkers for the construction of biosensors. This review focuses on the development for the detection of various biomarkers using multiple biosensing methods. Here, we describe the origin and the significance of OC-associated biomarkers, the working principle of biosensors, and the classification of biosensors based on their recognition elements and signal transducers. The modes of detection and sensitivity of the sensors are discussed. Finally, the challenges in the fabrication, obstacles in the clinical application, and future prospects are discussed.
Keywords: biosensors; cancer biomarkers; cancer diagnosis; ovarian cancer (OC).
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


Similar articles
-
A perspective on the potential use of aptamer-based field-effect transistor sensors as biosensors for ovarian cancer biomarkers CA125 and HE4.Talanta. 2025 Sep 1;292:127954. doi: 10.1016/j.talanta.2025.127954. Epub 2025 Mar 15. Talanta. 2025. PMID: 40120511 Review.
-
Early stage detection and screening of ovarian cancer: A research opportunity and significant challenge for biosensor technology.Biosens Bioelectron. 2019 Jun 15;135:71-81. doi: 10.1016/j.bios.2019.03.041. Epub 2019 Mar 20. Biosens Bioelectron. 2019. PMID: 31003031 Review.
-
Recent advances in surface plasmon resonance for the detection of ovarian cancer biomarkers: a thorough review.Mikrochim Acta. 2024 Oct 9;191(11):659. doi: 10.1007/s00604-024-06740-3. Mikrochim Acta. 2024. PMID: 39382786 Review.
-
Current and emerging biomarkers in ovarian cancer diagnosis; CA125 and beyond.Adv Protein Chem Struct Biol. 2023;133:85-114. doi: 10.1016/bs.apcsb.2022.08.003. Epub 2022 Sep 28. Adv Protein Chem Struct Biol. 2023. PMID: 36707207
-
The role of urine and serum biomarkers in the early detection of ovarian epithelial tumours.J Obstet Gynaecol. 2022 Nov;42(8):3441-3449. doi: 10.1080/01443615.2022.2151352. Epub 2023 Feb 9. J Obstet Gynaecol. 2022. PMID: 36757337 Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

