Recent Advances in Enzymatic Biofuel Cells to Power Up Wearable and Implantable Biosensors
- PMID: 40277532
- PMCID: PMC12024621
- DOI: 10.3390/bios15040218
Recent Advances in Enzymatic Biofuel Cells to Power Up Wearable and Implantable Biosensors
Abstract
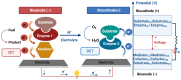
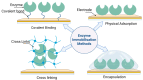
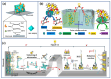
Enzymatic biofuel cells (EBFCs) have emerged as a transformative solution in the quest for sustainable energy, offering a biocatalyst-driven alternative for powering wearable and implantable self-powered biosensors. These systems harness renewable enzyme activity under mild conditions, positioning them as ideal candidates for next-generation biosensing applications. Despite their promise, their practical deployment is limited by challenges such as low power density, restricted operational lifespan, and miniaturization complexities. This review provides an in-depth exploration of the evolving landscape of EBFC technology, beginning with fundamental principles and the latest developments in electron transfer mechanisms. A critical assessment of enzyme immobilization techniques, including physical adsorption, covalent binding, entrapment, and cross-linking, underscores the importance of optimizing enzyme stability and catalytic activity for enhanced bioelectrode performance. Additionally, we examine advanced bioelectrode materials, focusing on the role of nanostructures such as carbon-based nanomaterials, noble metals, conducting polymers, and metal-organic frameworks in improving electron transfer and boosting biosensor efficiency. Also, this review includes case studies of EBFCs in wearable self-powered biosensors, with particular attention to the real-time monitoring of neurotransmitters, glucose, lactate, and ethanol through sweat analysis, as well as their integration into implantable devices for continuous healthcare monitoring. Moreover, a dedicated discussion on challenges and trends highlights key limitations, including durability, power management, and scalability, while presenting innovative approaches to address these barriers. By addressing both technical and biological constraints, EBFCs hold the potential to revolutionize biomedical diagnostics and environmental monitoring, paving the way for highly efficient, autonomous biosensing platforms.
Keywords: energy sources; enzymatic biofuel cells; implantable sensors; remote sensing; self-powered biosensors; wearable devices.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures







Similar articles
-
Wearable biofuel cells based on the classification of enzyme for high power outputs and lifetimes.Biosens Bioelectron. 2019 Jan 15;124-125:40-52. doi: 10.1016/j.bios.2018.09.086. Epub 2018 Sep 27. Biosens Bioelectron. 2019. PMID: 30343155 Review.
-
Fueling the Future: The Emergence of Self-Powered Enzymatic Biofuel Cell Biosensors.Biosensors (Basel). 2024 Jun 24;14(7):316. doi: 10.3390/bios14070316. Biosensors (Basel). 2024. PMID: 39056592 Free PMC article. Review.
-
Enzymatic Biofuel Cells on Porous Nanostructures.Small. 2016 Sep;12(34):4649-61. doi: 10.1002/smll.201600906. Epub 2016 Jul 5. Small. 2016. PMID: 27377976 Review.
-
Wearable Bioelectronics: Enzyme-Based Body-Worn Electronic Devices.Acc Chem Res. 2018 Nov 20;51(11):2820-2828. doi: 10.1021/acs.accounts.8b00451. Epub 2018 Nov 6. Acc Chem Res. 2018. PMID: 30398344 Free PMC article.
-
Ethanol Biofuel Cells: Hybrid Catalytic Cascades as a Tool for Biosensor Devices.Biosensors (Basel). 2021 Feb 4;11(2):41. doi: 10.3390/bios11020041. Biosensors (Basel). 2021. PMID: 33557146 Free PMC article. Review.
References
-
- Liu Z., Yang J., Wang H., Zhang J., Bai H., Peng B., Ai W., Du H., Li L., Chen P. Recent Progress in Mitochondrial Biofuel Cells. J. Electroanal. Chem. 2023;950:117881. doi: 10.1016/j.jelechem.2023.117881. - DOI
-
- Rewatkar P., Hitaishi V.P., Lojou E., Goel S. Enzymatic Fuel Cells in a Microfluidic Environment: Status and Opportunities. A Mini Review. Electrochem. Commun. 2019;107:106533. doi: 10.1016/j.elecom.2019.106533. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

