A Review on Optical Biosensors for Monitoring of Uric Acid and Blood Glucose Using Portable POCT Devices: Status, Challenges, and Future Horizons
- PMID: 40277536
- PMCID: PMC12025047
- DOI: 10.3390/bios15040222
A Review on Optical Biosensors for Monitoring of Uric Acid and Blood Glucose Using Portable POCT Devices: Status, Challenges, and Future Horizons
Abstract
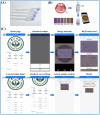
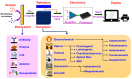
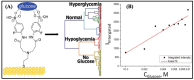
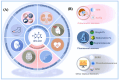
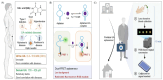
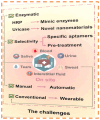
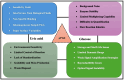
The growing demand for real-time, non-invasive, and cost-effective health monitoring has driven significant advancements in portable point-of-care testing (POCT) devices. Among these, optical biosensors have emerged as promising tools for the detection of critical biomarkers such as uric acid (UA) and blood glucose. Different optical transduction methods, like fluorescence, surface plasmon resonance (SPR), and colorimetric approaches, are talked about, with a focus on how sensitive, specific, and portable they are. Despite considerable advancements, several challenges persist, including sensor stability, miniaturization, interference effects, and the need for calibration-free operation. This review also explores issues related to cost-effectiveness, data integration, and wireless connectivity for remote monitoring. The review further examines regulatory considerations and commercialization aspects of optical biosensors, addressing the gap between research developments and clinical implementation. Future perspectives emphasize the integration of artificial intelligence (AI) and healthcare for improved diagnostics, alongside the development of wearable and implantable biosensors for continuous monitoring. Innovative optical biosensors have the potential to change the way people manage their health by quickly and accurately measuring uric acid and glucose levels. This is especially true as the need for decentralized healthcare solutions grows. By critically evaluating existing work and exploring the limitations and opportunities in the field, this review will help guide the development of more efficient, accessible, and reliable POCT devices that can improve patient outcomes and quality of life.
Keywords: blood glucose; optical biosensors; uric acid; μPADs.
Conflict of interest statement
None declared. All authors had full access to all the study data, and take responsibility for the integrity of the data and accuracy of the data analysis.
Figures









References
-
- Huang X., Xu D., Chen J., Liu J., Li Y., Song J., Ma X., Guo J. Smartphone-based analytical biosensors. Analyst. 2018;143:5339–5351. - PubMed
-
- Lino C., Barrias S., Chaves R., Adega F., Martins-Lopes P., Fernandes J.R. Biosensors as diagnostic tools in clinical applications. Biochim. Biophys. Acta Rev. Cancer. 2022;1877:188726. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

