Aptamers as Diagnostic and Therapeutic Agents for Aging and Age-Related Diseases
- PMID: 40277546
- PMCID: PMC12024714
- DOI: 10.3390/bios15040232
Aptamers as Diagnostic and Therapeutic Agents for Aging and Age-Related Diseases
Abstract
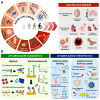
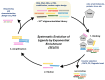
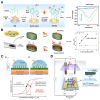
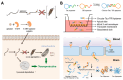
In the 21st century, the demographic shift toward an aging population has posed a significant challenge, particularly with respect to age-related diseases, which constitute a major threat to human health. Accordingly, the detection, prevention, and treatment of aging and age-related diseases have become critical issues, and the introduction of novel molecular recognition elements, called aptamers, has been considered. Aptamers, a class of oligonucleotides, can bind to target molecules with high specificity. In addition, aptamers exhibit superior stability, biocompatibility, and applicability, rendering them promising tools for the diagnosis and treatment of human diseases. In this paper, we present a comprehensive overview of aptamers, systematic evolution of ligands by exponential enrichment (SELEX), biomarkers associated with aging, as well as aptamer-based diagnostic and therapeutic platforms. Finally, the limitations associated with predicting and preventing age-related conditions are discussed, along with potential solutions based on advanced technologies and theoretical approaches.
Keywords: age-related disease; aging; aptamer; circadian rhythm; diagnosis; therapeutics.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Similar articles
-
Aptamers used for biosensors and targeted therapy.Biomed Pharmacother. 2020 Dec;132:110902. doi: 10.1016/j.biopha.2020.110902. Epub 2020 Oct 20. Biomed Pharmacother. 2020. PMID: 33096353 Free PMC article.
-
Recent advancements in aptamers as promising nanotool for therapeutic and diagnostic applications.Anal Biochem. 2025 Jul;702:115844. doi: 10.1016/j.ab.2025.115844. Epub 2025 Mar 14. Anal Biochem. 2025. PMID: 40090606 Review.
-
Generating aptamers by cell-SELEX for applications in molecular medicine.Int J Mol Sci. 2012;13(3):3341-3353. doi: 10.3390/ijms13033341. Epub 2012 Mar 12. Int J Mol Sci. 2012. PMID: 22489154 Free PMC article. Review.
-
Advances in Aptamer-Based Biosensors and Cell-Internalizing SELEX Technology for Diagnostic and Therapeutic Application.Biosensors (Basel). 2022 Oct 25;12(11):922. doi: 10.3390/bios12110922. Biosensors (Basel). 2022. PMID: 36354431 Free PMC article. Review.
-
Viral aptamer screening and aptamer-based biosensors for virus detection: A review.Int J Biol Macromol. 2024 Sep;276(Pt 2):133935. doi: 10.1016/j.ijbiomac.2024.133935. Epub 2024 Jul 17. Int J Biol Macromol. 2024. PMID: 39029851 Review.
References
-
- United Nations World Population Prospects 2024: Summary of Results. [(accessed on 1 January 2025)]. Available online: https://population.un.org/wpp/assets/Files/WPP2024_Summary-of-Results.pdf.
-
- de Magalhaes J.P. Distinguishing between driver and passenger mechanisms of aging. Nat. Genet. 2024;56:204–211. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

