White Light Spectroscopy Characteristics and Expansion Dynamic Behavior of Primary T-Cells: A Possibility of Online, Real-Time, and Sampling-Less CAR T-Cell Production Monitoring
- PMID: 40277564
- PMCID: PMC12025026
- DOI: 10.3390/bios15040251
White Light Spectroscopy Characteristics and Expansion Dynamic Behavior of Primary T-Cells: A Possibility of Online, Real-Time, and Sampling-Less CAR T-Cell Production Monitoring
Abstract
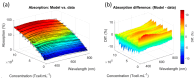
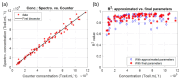
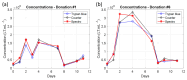
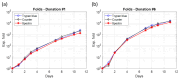
The production of advanced therapy medicinal products (ATMP) is a long and highly technical process, resulting in a high cost per dose, which reduces the number of eligible patients. There is a critical need for a closed and sample-free monitoring system to perform the numerous quality controls required. Current monitoring methods are not optimal, mainly because they require the system to be opened up for sampling and result in material losses. White light spectroscopy has emerged as a technique for sample-free control compatible with closed systems. We have recently proposed its use to monitor cultures of CEM-C1 cell lines. In this paper, we apply this method to T-cells isolated from healthy donor blood samples. The main differences between cell lines and human primary T-cells lie in the slightly different shape of their absorption spectra and in the dynamics of cell expansion. T-cells do not multiply exponentially, resulting in a non-constant generation time. Cell expansion is described by a power-law model, which allows for the definition of instantaneous generation times. A correlation between the linear asymptotic behavior of these generation times and the initial cell concentration leads to the hypothesis that this could be an early predictive marker of the final culture concentration. To the best of our knowledge, this is the first time that such concepts have been proposed.
Keywords: CAR T-cell; advanced therapy medicinal product; cell concentration monitoring; quality control; white light spectroscopy.
Conflict of interest statement
There are no conflicts of interest.
Figures



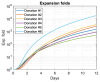

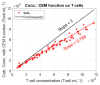

References
-
- ATMP: Advanced Therapy Medicinal Product European Medicines Agency. [(accessed on 4 December 2024)]. Available online: https://www.ema.europa.eu/en/human-regulatory-overview/advanced-therapy-....
-
- Wacogne B., Legrand D., Azzopardi C.L., Pieralli C., Frelet-Barrand A. Optical Spectroscopy Methods to Monitor Cells and Bacteria Concentrations and to Detect Contamination during Cell Culture: Application to the Fabrication of ATMPs. In: Ye X., Soares F., De Maria E., Comez Vilda P., Cabitza F., Fred A., Gamboa H., editors. Biomedical Engineering Systems and Technologies. Communications in Computer and Information Science. Volume 1400. Springer Nature; Cham, Switzerland: 2021. pp. 53–75. (Springer Book Series).
-
- Wacogne B., Legrand D., Pieralli C., Frelet-Barrand A. Optical Spectroscopy for the Quality Control of ATMP Fabrication: A New Method to Monitor Cell Expansion and to Detect Contaminations; Proceedings of the 13th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2020)—BIODEVICES; Valletta, Malta. 24–26 February 2020; pp. 64–72.
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

