A Systematic Review of Cost-Effectiveness Studies on Pancreatic Cancer Screening
- PMID: 40277782
- PMCID: PMC12025814
- DOI: 10.3390/curroncol32040225
A Systematic Review of Cost-Effectiveness Studies on Pancreatic Cancer Screening
Abstract
Background: Pancreatic cancer (PC) is among the deadliest types of cancer globally. While early detection helps avert adverse outcomes, screening is only recommended for individuals at high risk, specifically those with familial and/or genetic predispositions. The objectives of this study are to systematically review primary studies on the cost-effectiveness of PC screening and to identify the critical factors that influence cost-effectiveness.
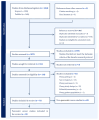
Methods: This systematic review was performed using PRISMA guidelines. Economic evaluation studies on PC screening were identified from searches on the SCOPUS and PubMed databases. The quality of reporting of the selected articles was assessed according to CHEERS 2022. Using predefined inclusion and exclusion criteria, two reviewers conducted the title-abstract review, full-text review, and data extraction to select relevant articles. The authors' consensus was used to settle disagreements. The primary outcome was the incremental cost-effectiveness ratio, measured by cost per quality-adjusted life year and cost per life year saved.
Results: Nine studies were selected for the final review. Most studies demonstrated that one-time screening for PC among high-risk individuals was cost-effective compared with no screening, while others found annual screening to also be cost-effective. High-risk was generally defined as having a >5% lifetime risk of PC and included individuals with either familial pancreatic cancer (FPC) or genetic susceptibility syndromes such as Peutz-Jeghers Syndrome, hereditary pancreatitis, hereditary non-polypoid colorectal cancer syndrome, familial adenomatous polyposis, and BRCA2 mutations. Individuals with new-onset diabetes (NOD) were also considered high-risk. Screening using mainly endoscopic ultrasound was cost-effective among FPC individuals and those with genetic syndromes. Risk-based screening was also cost-effective among patients with NOD.
Conclusion: Screening for PC is cost-effective among selected high-risk individuals. However, cost-effectiveness depends on epidemiological factors, cost, the diagnostic performance of screening tools, and the overall design of studies.
Keywords: cost-effectiveness; pancreatic cancer screening; systematic review.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of topotecan for ovarian cancer.Health Technol Assess. 2001;5(28):1-110. doi: 10.3310/hta5280. Health Technol Assess. 2001. PMID: 11701100
-
Adefovir dipivoxil and pegylated interferon alfa-2a for the treatment of chronic hepatitis B: a systematic review and economic evaluation.Health Technol Assess. 2006 Aug;10(28):iii-iv, xi-xiv, 1-183. doi: 10.3310/hta10280. Health Technol Assess. 2006. PMID: 16904047
-
Cost-effectiveness of using prognostic information to select women with breast cancer for adjuvant systemic therapy.Health Technol Assess. 2006 Sep;10(34):iii-iv, ix-xi, 1-204. doi: 10.3310/hta10340. Health Technol Assess. 2006. PMID: 16959170
-
The effectiveness and cost-effectiveness of carmustine implants and temozolomide for the treatment of newly diagnosed high-grade glioma: a systematic review and economic evaluation.Health Technol Assess. 2007 Nov;11(45):iii-iv, ix-221. doi: 10.3310/hta11450. Health Technol Assess. 2007. PMID: 17999840
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of paclitaxel, docetaxel, gemcitabine and vinorelbine in non-small-cell lung cancer.Health Technol Assess. 2001;5(32):1-195. doi: 10.3310/hta5320. Health Technol Assess. 2001. PMID: 12065068
References
-
- Ferlay J., Ervik M., Lam F., Laversanne M., Colombet M., Mery L., Piñeros M., Znaor A., Soerjomataram I. Bray Global Cancer Observatory: Cancer Today. [(accessed on 17 February 2024)]. Available online: https://gco.iarc.who.int/today.
-
- Huang L., Jansen L., Balavarca Y., Molina-Montes E., Babaei M., van der Geest L., Lemmens V., Van Eycken L., De Schutter H., Johannesen T.B., et al. Resection of Pancreatic Cancer in Europe and USA: An International Large-Scale Study Highlighting Large Variations. Gut. 2019;68:130–139. doi: 10.1136/gutjnl-2017-314828. - DOI - PubMed
-
- National Cancer Institute SEER Cancer Stat Facts: Pancreatic Cancer. [(accessed on 22 September 2024)]; Available online: https://seer.cancer.gov/statfacts/html/pancreas.html.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous