Novel Thyroid Hormone Receptor-β Agonist TG68 Exerts Anti-Inflammatory, Lipid-Lowering and Anxiolytic Effects in a High-Fat Diet (HFD) Mouse Model of Obesity
- PMID: 40277905
- PMCID: PMC12026167
- DOI: 10.3390/cells14080580
Novel Thyroid Hormone Receptor-β Agonist TG68 Exerts Anti-Inflammatory, Lipid-Lowering and Anxiolytic Effects in a High-Fat Diet (HFD) Mouse Model of Obesity
Abstract
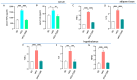
Recent advances in drug development allowed for the identification of THRβ-selective thyromimetic TG68 as a very promising lipid lowering and anti-amyloid agent. In the current study, we first investigated the neuroprotective effects of TG68 on in vitro human models of neuroinflammation and β-amyloid neurotoxicity in order to expand our knowledge of the therapeutic potential of this novel thyromimetic. Subsequently, we examined metabolic and inflammatory profiles, along with cognitive changes, using a high-fat diet (HFD) mouse model of obesity. Our data demonstrated that TG68 was able to prevent either LPS/TNFα-induced inflammatory response or β-amyloid-induced cytotoxicity in human microglial (HMC3) cells. Next, we demonstrated that in HFD-fed mice, treatment with TG68 (10 mg/kg/day; 2 weeks) significantly reduced anxiety-like behavior in stretch-attend posture (SAP) tests while producing a 12% BW loss and a significant decrease in blood glucose and lipid levels. Notably, these data highlight a close relationship between improved serum metabolic parameters and a reduction of anxious behavior. Moreover, TG68 administration was observed to efficiently counteract HFD-altered central and peripheral expressions in mice with selected biomarkers of metabolic dysfunction, inflammation, and neurotoxicity, revealing promising neuroprotective effects. In conclusion, our work provides preliminary evidence that TG68 may represent a novel therapeutic opportunity for the treatment of interlinked diseases such as obesity and neurodegenerative diseases.
Keywords: anxiety; microglia; neurodegeneration; neuroinflammation; obesity; thyromimetics.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




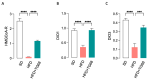




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

