Neuroprotection by Mitochondrial NAD Against Glutamate-Induced Excitotoxicity
- PMID: 40277908
- PMCID: PMC12025592
- DOI: 10.3390/cells14080582
Neuroprotection by Mitochondrial NAD Against Glutamate-Induced Excitotoxicity
Abstract
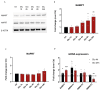
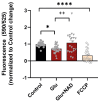
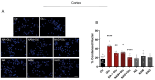
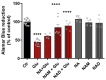
Excitotoxicity is a pathological process that occurs in many neurological diseases, such as stroke or epilepsy, and is characterized by the extracellular accumulation of high concentrations of glutamate or other excitatory amino acids (EAAs). Nicotinamide adenine dinucleotide (NAD) depletion is an early event following excitotoxicity in many in vitro and in vivo excitotoxic-related models and contributes to the deregulation of energy homeostasis. However, the interplay between glutamate excitotoxicity and the NAD biosynthetic pathway is not fully understood. To address this question, we used a primary culture of rat cortical neurons and found that an excitotoxic glutamate insult alters the expression of the NAD biosynthetic enzymes. Additionally, using a fluorescent NAD mitochondrial sensor, we observed that glutamate induces a significant decrease in the mitochondrial NAD pool, which was reversed when exogenous NAD was added. We also show that exogenous NAD protects against the glutamate-induced decrease in mitochondrial membrane potential (MMP). Glutamate excitotoxicity changed mitochondrial retrograde transport in neurites, which seems to be reversed by NAD addition. Finally, we show that NAD and NAD precursors protect against glutamate-induced cell death. Together, our results demonstrate that glutamate-induced excitotoxicity acts by compromising the NAD biosynthetic pathway, particularly in the mitochondria. These results also uncover a potential role for mitochondrial NAD as a tool for central nervous system (CNS) regenerative therapies.
Keywords: NAD metabolism; excitotoxicity; glutamate; mitochondria.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

