LRG1 Alters Pericyte Phenotype and Compromises Vascular Maturation
- PMID: 40277918
- PMCID: PMC12026257
- DOI: 10.3390/cells14080593
LRG1 Alters Pericyte Phenotype and Compromises Vascular Maturation
Abstract
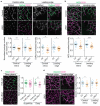
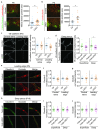
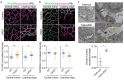
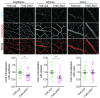
Upregulation of leucine-rich alpha-2-glycoprotein-1 (LRG1) contributes to aberrant neovascularization in many different diseases. In contrast, LRG1 is not involved in developmental angiogenesis. Here, we investigated the vasculopathic properties of LRG1 by examining its effect on developing retinal blood vessels. By injecting recombinant protein or an expression vector into the mouse retina during vascular development, we showed that exogenous LRG1 reduces pericyte coverage and NG2 expression. It leads to diminished collagen IV sheathing, fewer adhesion and gap junctions, and reduced vessel calibre and vascular density. Moreover, in mouse retinae containing exogenous LRG1, the developing blood-retinal barrier remains more permeable with significantly higher numbers of transcytotic vesicles present in microvascular endothelial cells. These results reveal that exogeneous LRG1 is sufficient to interfere with the maturation of developing retinal vessels and drive vessel development towards a dysfunctional phenotype. These observations deliver further evidence that LRG1 is an angiopathic factor and highlight the therapeutic potential of blocking LRG1 in diseases characterized by pathogenic angiogenesis or vascular remodelling.
Keywords: angiogenesis; blood vessel maturation; blood–retinal barrier; leucine-rich alpha-2-glycoprotein-1 (LRG1); pericyte.
Conflict of interest statement
J.G. and S.E.M. are founders and members of the scientific advisory board of a company spun out by UCL Business to commercialize a LRG1 function-blocking therapeutic antibody developed through the UK Medical Research Council DPFS funding scheme. J.G. and S.E.M. are shareholders of this company and named inventors on three patents related to LRG1 as a therapeutic target. All other authors have declared that no conflicts of interest exist.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
- National Institute for Health Research (NIHR) Biomedical Research Centre based at Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology
- 18/0005856/DUK_/Diabetes UK/United Kingdom
- GR001145/Moorfields Eye Charity
- ST1507E/Moorfields Eye Charity
- PG/20/20/35060/BHF_/British Heart Foundation/United Kingdom
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

