MMP3 as a Molecular Link: Unraveling the Connection Between Ankylosing Spondylitis and Acute Coronary Syndrome
- PMID: 40277922
- PMCID: PMC12025634
- DOI: 10.3390/cells14080597
MMP3 as a Molecular Link: Unraveling the Connection Between Ankylosing Spondylitis and Acute Coronary Syndrome
Abstract
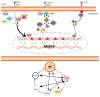
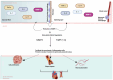
Ankylosing spondylitis (AS) is a chronic inflammatory disease that primarily affects the joints, limiting patients' mobility and quality of life. Recent studies have shown that patients with AS have a significantly higher risk of developing severe cardiovascular complications, such as acute coronary syndrome (ACS). A comprehensive review (2014-2024) included a study evaluating the significance of matrix metalloproteinase 3 (MMP-3) in cardiovascular risk among AS patients. The findings indicate that chronic inflammation in AS not only damages the joints but also contributes to the progression of cardiovascular diseases. At the molecular level, MMP-3 is instrumental in degrading the extracellular matrix, leading to instability in the atherosclerotic plaques and increasing the risk of ACS. Additionally, MMP-3 activation is related to the inflammatory pathways, such as tumor necrosis factor-alpha (TNF-α) and NF-κB, which amplify its effect on both joint destruction and vascular damage. This molecular approach offers new perspectives for understanding and treating AS and its cardiovascular complications, suggesting that MMP-3 inhibition could be a promising therapeutic strategy to mitigate cardiovascular risk in these patients.
Keywords: acute coronary syndrome; ankylosing spondylitis; cardiovascular risk; chronic inflammation; extracellular matrix degradation; matrix metalloproteinase 3 (MMP-3).
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
References
-
- Navarini L., Caso F., Costa L., Currado D., Stola L., Perrotta F., Delfino L., Sperti M., Deriu M.A., Ruscitti P., et al. Cardiovascular risk prediction in ankylosing spondylitis: From traditional scores to machine learning assessment. Rheumatol. Ther. 2020;7:867–882. doi: 10.1007/s40744-020-00233-4. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

