Deciphering the Controversial Role of TP53 Inducible Glycolysis and Apoptosis Regulator (TIGAR) in Cancer Metabolism as a Potential Therapeutic Strategy
- PMID: 40277923
- PMCID: PMC12025843
- DOI: 10.3390/cells14080598
Deciphering the Controversial Role of TP53 Inducible Glycolysis and Apoptosis Regulator (TIGAR) in Cancer Metabolism as a Potential Therapeutic Strategy
Abstract
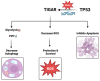
Tumor metabolism has emerged as a critical target in cancer therapy, revolutionizing our understanding of how cancer cells grow, survive, and respond to treatment. Historically, cancer research focused on genetic mutations driving tumorigenesis, but in recent decades, metabolic reprogramming has been recognized as a hallmark of cancer. The TP53 inducible glycolysis and apoptosis regulator, or TIGAR, affects a wide range of cellular and molecular processes and plays a key role in cancer cell metabolism by regulating the balance between glycolysis and antioxidant defense mechanisms. Cancer cells often exhibit a shift towards aerobic glycolysis (the Warburg effect), which allows rapid energy production and gives rise to biosynthetic intermediates for proliferation. By inhibiting glycolysis, TIGAR can reduce the proliferation rate of cancer cells, particularly in early-stage tumors or specific tissue types. This metabolic shift may limit the resources available for rapid cell division, thereby exerting a tumor-suppressive effect. However, this metabolic shift also leads to increased levels of reactive oxygen species (ROS), which can damage the cell if not properly managed. TIGAR helps protect cancer cells from excessive ROS by promoting the pentose phosphate pathway (PPP), which generates NADPH-a key molecule involved in antioxidant defense. Through its actions, TIGAR decreases the glycolytic flux while increasing the diversion of glucose-6-phosphate into the PPP. This reduces ROS levels and supports biosynthesis and cell survival by maintaining the balance of nucleotides and lipids. The role of TIGAR has been emerging as a prognostic and potential therapeutic target in different types of cancers. This review highlights the role of TIGAR in different types of cancer, evaluating its potential role as a diagnostic marker and a therapeutic target.
Keywords: P53; ROS; cancer metabolism; gastric cancer; ketogenic diet; pancreatic cancer; pentose phosphate pathway.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Inhibition of TIGAR Increases Exogenous p53 and Cisplatin Combination Sensitivity in Lung Cancer Cells by Regulating Glycolytic Flux.Int J Mol Sci. 2022 Dec 16;23(24):16034. doi: 10.3390/ijms232416034. Int J Mol Sci. 2022. PMID: 36555672 Free PMC article.
-
Tp53-induced glycolysis and apoptosis regulator (TIGAR) protects glioma cells from starvation-induced cell death by up-regulating respiration and improving cellular redox homeostasis.J Biol Chem. 2012 Sep 28;287(40):33436-46. doi: 10.1074/jbc.M112.384578. Epub 2012 Aug 10. J Biol Chem. 2012. PMID: 22887998 Free PMC article.
-
p53-TP53-Induced Glycolysis Regulator Mediated Glycolytic Suppression Attenuates DNA Damage and Genomic Instability in Fanconi Anemia Hematopoietic Stem Cells.Stem Cells. 2019 Jul;37(7):937-947. doi: 10.1002/stem.3015. Epub 2019 May 3. Stem Cells. 2019. PMID: 30977208 Free PMC article.
-
The diverse role of TIGAR in cellular homeostasis and cancer.Free Radic Res. 2018 Dec;52(11-12):1240-1249. doi: 10.1080/10715762.2018.1489133. Epub 2018 Oct 4. Free Radic Res. 2018. PMID: 30284488 Review.
-
Structure, regulation, and biological functions of TIGAR and its role in diseases.Acta Pharmacol Sin. 2021 Oct;42(10):1547-1555. doi: 10.1038/s41401-020-00588-y. Epub 2021 Jan 28. Acta Pharmacol Sin. 2021. PMID: 33510458 Free PMC article. Review.
Cited by
-
Unlocking the Role of Metabolic Pathways in Brain Metastatic Disease.Cells. 2025 May 13;14(10):707. doi: 10.3390/cells14100707. Cells. 2025. PMID: 40422210 Free PMC article. Review.
References
-
- Stincone A., Prigione A., Cramer T., Wamelink M.M.C., Campbell K., Cheung E., Olin-Sandoval V., Grüning N.-M., Krüger A., Tauqeer Alam M., et al. The return of metabolism: Biochemistry and physiology of the pentose phosphate pathway. Biol. Rev. 2015;90:927–963. doi: 10.1111/brv.12140. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous