Mimicking the Liver Sinusoidal Endothelial Cell Niche In Vitro to Enhance Fenestration in a Genetic Model of Systemic Inflammation
- PMID: 40277946
- PMCID: PMC12025456
- DOI: 10.3390/cells14080621
Mimicking the Liver Sinusoidal Endothelial Cell Niche In Vitro to Enhance Fenestration in a Genetic Model of Systemic Inflammation
Abstract
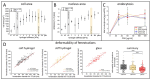
Liver sinusoidal endothelial cells (LSECs) play a crucial role in hepatic homeostasis, clearance, and microcirculatory regulation. Their fenestrations-patent transcellular pores-are essential for proper liver function, yet disappear in pathological conditions such as liver fibrosis and inflammation through a process known as defenestration. Defenestrated sinusoids are often linked to the liver stiffening that occurs through mechanotransduction-regulated processes. We performed a detailed characterization of polyacrylamide (PAA) hydrogels using atomic force microscopy (AFM), rheometry, scanning electron microscopy, and fluorescence microscopy to assess their potential as biomimetic substrates for LSECs. We additionally implemented AFM; quantitative fluorescence microscopy, including high-resolution structured illumination microscopy (HR-SIM); and an endocytosis assay to characterize the morphology and function of LSECs. Our results revealed significant local variations in hydrogel stiffness and differences in pore sizes. The primary LSECs cultured on these substrates had a range of stiffnesses and were analyzed with regard to their number of fenestrations, cytoskeletal organization, and endocytic function. To explore mechanotransduction in inflammatory liver disease, we investigated LSECs from a genetic model of systemic inflammation triggered by the deletion of Mcpip1 in myeloid leukocytes and examined their ability to restore their fenestrations on soft substrates. Our study demonstrates the beneficial effect of soft hydrogels on LSECs. Control cells exhibited a similar fenestrated morphology and function compared to cells cultured on plastic substrates. However, the pathological LSECs from the genetic model of systemic inflammation regained their fenestrations when cultured on soft hydrogels. This observation supports previous findings on the beneficial effects of soft substrates on LSEC fenestration status.
Keywords: actin cytoskeleton; atomic force microscopy; elastic properties; fenestrations; liver sinusoidal endothelial cells; mechanotransduction; polyacrylamide.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
- UMO-2019/35/D/NZ3/01804/Polish National Science Centre
- No 101119613/European Union's Horizon research and innovation programme under the Marie Skłodow-ska-Curie project ImAgE-D
- No. 101046928/European Union's European Innovation Council (EIC) PATHFINDER Open Programme, project DeLIVERy and the Hop-On Facility HORIZON-WIDERA program associated with DeLIVERy
LinkOut - more resources
Full Text Sources
Miscellaneous

