Non-Adherence Rate to Oral Mesalamine in Ulcerative Colitis Patients: A Systematic Review with Meta-Analysis
- PMID: 40278302
- PMCID: PMC12028940
- DOI: 10.3390/jpm15040123
Non-Adherence Rate to Oral Mesalamine in Ulcerative Colitis Patients: A Systematic Review with Meta-Analysis
Abstract
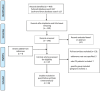
Background/Objectives: Ulcerative colitis (UC) is a part of inflammatory bowel disease (IBD) and it is characterized by colonic-mucosal chronic inflammation with intermittent clinical activity. Personalized medicine is becoming more and more a relevant method of approach in this field, and the identification of potential concerns in a single patient may contribute to the improvement of the clinical approach. Mesalamine represents the cornerstone of therapy for mild-moderate disease forms, but non-adherence to medical therapy represents a critical health problem, although it is underestimated by many physicians, with evident consequences in terms of disease-related complications. The aim of the present study is to evaluate the magnitude of non-adherence to oral mesalamine in UC patients performing a systematic review and meta-analysis of literature. Methods: A literature search in PubMed and Cochrane databases was performed for studies reporting the non-adherence rate to oral mesalamine in adult UC patients, and eligible studies have been selected for evaluation. The type of study (trial vs. observational), geographic area, sample size, method of adherence assessment, and non-adherence rate were considered. Results: From a total of 464 articles, 34 studies were included in the meta-analysis after selection. Sixteen studies (47%) are observational, and eighteen (53%) are clinical trials. A total of 12/34 (35%) studies are from North America, 14/34 (41%) from Europe, 4/34 (12%) from Asia, with 4/34 (12%) from mixed areas of the world. The mean non-adherence rate was 32%, but with a consistent variability among the studies. In particular, the non-adherence rate was significantly higher in observational studies vs. clinical trials (47 vs. 20%, p < 0.001), and in North American vs. European and Asian studies (54 vs. 23 vs. 4%, respectively, p < 0.001). Conclusions: The non-adherence rate to oral mesalamine is variably reported in the literature due to the inhomogeneity of available studies, but it represents a consistent problem, often neglected, that deserves future research. A personalized approach by a physician to a single patient can improve the effectiveness of medical therapy and the management of UC patients.
Keywords: adherence; inflammatory bowel disease; mesalamine; ulcerative colitis.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Sabaté E. Adherence to Long-Term Therapies: Evidence for Action. World Health Organization; Geneva, Switzerland: 2003.
Publication types
LinkOut - more resources
Full Text Sources

