Enzyme-Linked Immunosorbent Assay (ELISA) Development for Equine Serum Amyloid A (SAA) Determination Using Recombinant Proteins
- PMID: 40278511
- PMCID: PMC12029847
- DOI: 10.3390/mps8020037
Enzyme-Linked Immunosorbent Assay (ELISA) Development for Equine Serum Amyloid A (SAA) Determination Using Recombinant Proteins
Abstract
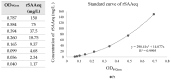
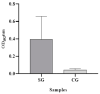
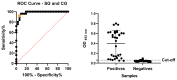
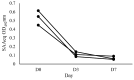
We aimed to develop a species-specific ELISA for qualitatively and quantitatively determining serum amyloid A (SAA) in horses. Current methods for measuring SAA in horses utilize ELISA or immunoturbidimetric tests designed for human SAA, which are not specific to horses. Mice and rabbits were used to generate polyclonal antibodies against equine SAA. The study examined serum samples from 32 horses with acute inflammatory disease (SG) and 25 clinically healthy horses. Furthermore, the SAAeq kinetics were observed in three horses from the SG group at three different timepoints. The SAA-ELISA established a cut-off at 0.06 OD492nm, where values equal to or higher than this were deemed positive, while values below it was considered negative. The test exhibited a sensitivity of 94% and specificity of 92%, resulting in an overall accuracy of 93%. The positive and negative predictive values were 94% and 92%, respectively. Coefficients of variation for inter- and intra-assay were 6.1% and 7.46% for SG and 9.6% and 9.63% for the control group (CG). The detection limit was determined to be 0.067. The SAA-ELISA proved its worth by demonstrating satisfactory performance, paving the way for the development of automated quantitative tests and species-specific semi-quantitative tests. This paves the way for their application in practical field settings.
Keywords: acute-phase; horse; immunoassay; inflammation; protein.
Conflict of interest statement
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures

Similar articles
-
Evaluation of an automated assay based on monoclonal anti-human serum amyloid A (SAA) antibodies for measurement of canine, feline, and equine SAA.Vet J. 2012 Dec;194(3):332-7. doi: 10.1016/j.tvjl.2012.05.007. Epub 2012 Jun 15. Vet J. 2012. PMID: 22704135
-
Validation of an equine serum amyloid A assay with an unusually broad working range.BMC Vet Res. 2019 Dec 19;15(1):462. doi: 10.1186/s12917-019-2211-3. BMC Vet Res. 2019. PMID: 31856804 Free PMC article.
-
Evaluation of a commercially available human serum amyloid A (SAA) turbidometric immunoassay for determination of equine SAA concentrations.Vet J. 2006 Sep;172(2):315-9. doi: 10.1016/j.tvjl.2005.04.021. Epub 2005 Jun 13. Vet J. 2006. PMID: 15950503
-
Serum amyloid A in equine health and disease.Equine Vet J. 2019 May;51(3):293-298. doi: 10.1111/evj.13062. Epub 2019 Feb 6. Equine Vet J. 2019. PMID: 30565319 Free PMC article. Review.
-
Use of serum amyloid A in equine medicine and surgery.Vet Clin Pathol. 2023 Feb;52 Suppl 1:8-18. doi: 10.1111/vcp.13195. Epub 2022 Nov 6. Vet Clin Pathol. 2023. PMID: 36336845 Review.
References
-
- Eckersall P.D. Clinical Biochemistry of Domestic Animals. 6th ed. Academic Press; San Diego, MA, USA: 2008. Proteins, Proteomics, and the Dysproteinemias; pp. 117–156.
-
- Kiemle J., Hindenberg S., Bauer N., Roecken M. Comparison of a point-of-care serum amyloid A analyzer frequently used in equine practice with 2 turbidimetric immunoassays used in human and veterinary medicine. J. Vet. Diagnostic Investig. 2022;34:42–53. doi: 10.1177/10406387211056029. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

