Prepubertal Exposure to Tris(2-chloroethyl) Phosphate Disrupts Blood-Testis Barrier Integrity via Ferritinophagy-Mediated Ferroptosis
- PMID: 40278601
- PMCID: PMC12031567
- DOI: 10.3390/toxics13040285
Prepubertal Exposure to Tris(2-chloroethyl) Phosphate Disrupts Blood-Testis Barrier Integrity via Ferritinophagy-Mediated Ferroptosis
Abstract
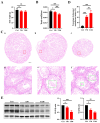
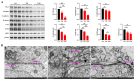
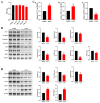
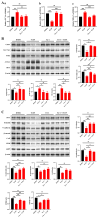
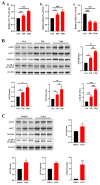
Tris(2-chloroethyl) phosphate (TCEP) is a representative chlorinated organophosphate flame retardant (OPFR) that demonstrates greater persistence than other non-halogenated alkyl or aryl OPFRs. Although TCEP has been shown to accumulate significantly in the environment and contribute to testicular toxicity and spermatogenic dysfunction, the precise underlying factors and mechanisms of action remain unclear. Herein, male ICR mice were gavaged with corn oil, 50 mg/kg body weight (bw) TCEP, or 100 mg/kg bw TCEP from postnatal day (PND) 22 to PND 35. TCEP exposure resulted in the disruption of blood-testis barrier (BTB) integrity and in abnormal testicular development. Considering that Sertoli cells constitute the primary target of toxicants and that TCEP induces oxidative stress in the testis and other organs, we focused on ferroptosis in Sertoli cells. Our findings revealed a significant increase in ferroptosis in the testes and Sertoli cells following TCEP exposure, and we observed functional restoration of Sertoli cell junctions upon treatment with the ferroptosis inhibitor ferrostatin-1. Furthermore, ferritin heavy chain 1 (FTH1) was markedly reduced in TCEP-exposed testes and Sertoli cells. Since nuclear receptor coactivator 4 (NCOA4)-mediated ferritinophagy is essential for the degradation of FTH1, we assessed ferritinophagic activity and found significant upregulation of NCOA4, ATG5, ATG7, and LC3B II/I in TCEP-exposed testes and Sertoli cells. These results strongly suggest that TCEP triggers Sertoli cell ferroptosis by activating ferritinophagy that leads to reduced expression of BTB-associated proteins, ultimately causing BTB disruption and testicular developmental toxicity.
Keywords: BTB; TCEP; ferritinophagy; ferroptosis.
Conflict of interest statement
Honglei Liu is an employee of Nanjing Shenghong Environmental Technology Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Fang B., Wang C., Du X., Sun G., Jia B., Liu X., Qu Y., Zhang Q., Yang Y., Li Y.Q., et al. Structure-dependent destructive adsorption of organophosphate flame retardants on lipid membranes. J. Hazard. Mater. 2024;478:135494. - PubMed
-
- Yang J., Li X., Yang H., Zhao W., Li Y. OPFRs in e-waste sites: Integrating in silico approaches, selective bioremediation, and health risk management of residents surrounding. J. Hazard. Mater. 2022;429:128304. - PubMed
-
- Ma H., He J., Fan H., Zhang N., Wu Q., Zhang S., Zhang C., Huang T., Gao H., Ma J., et al. The influence of emerging atmospheric organophosphorus flame retardants from land source emissions on the East China Sea. J. Hazard. Mater. 2024;465:133404. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

