Establishment and Comparison of Detection Methods for Ricin and Abrin Based on Their Depurination Activities
- PMID: 40278675
- PMCID: PMC12031163
- DOI: 10.3390/toxins17040177
Establishment and Comparison of Detection Methods for Ricin and Abrin Based on Their Depurination Activities
Abstract
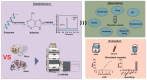
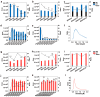
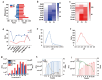
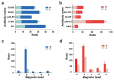
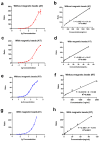
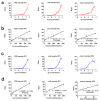
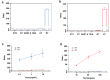
Ricin (RT) and abrin (AT) are plant toxins extracted from Ricinus communis and Abrus precatorius, respectively, and both have N-glycosidase activity. The detection of these toxins is vital because of their accessibility and bioterrorism potential. While ricin can be effectively detected based on its depurination activity, only a few tests are available for detecting the depurination activity of abrin. Therefore, it is unclear whether they share the same optimal reaction substrate and conditions. Here, we established optimum depurination conditions for ricin and abrin, facilitating the in vitro detection of their depurination activity using high-performance liquid chromatography-tandem mass spectrometry. The parameters optimized were the reaction substrate, bovine serum albumin (BSA), buffer, pH, temperature, time, antibodies, and magnetic beads. Both toxins showed better depurination with single-stranded DNA. However, substrate length, adenine content, BSA concentration, buffer concentration, reaction temperature, and reaction time differed between the two toxins. The optimal conditions for ricin depurination involved a reaction in 1 mM ammonium acetate solution (0.5 μM DNA15A, 20 μg/mL BSA, and 1 mM Zn2+, with pH 4.0) at 55 °C for 1 h. The optimal conditions for abrin depurination involved a reaction in 1 mM ammonium citrate solution (0.2 μM DNA20A, 10 μg/mL BSA, 1 mM Mg2+, and 0.5 mM EDTA, with pH 4.0) at 45 °C for 2 h. After optimization, the limits of detection (LOD) for ricin and abrin were 0.506 ng/mL and 0.168 ng/mL, respectively. The detection time was also significantly reduced.
Keywords: HPLC–MS/MS; N-glucosidase activity; abrin; detection; ricin.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Eric A.E.G. Toxicity and detection of ricin and abrin in beverages. J. Food Prot. 2008;71:1875–1883. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

