Feasibility of Pressurized Intraperitoneal Aerosol Chemotherapy (PIPAC) in a Rabbit Model of Peritoneal Metastases: PIPALIM Project
- PMID: 40279057
- PMCID: PMC12222299
- DOI: 10.1245/s10434-025-17251-7
Feasibility of Pressurized Intraperitoneal Aerosol Chemotherapy (PIPAC) in a Rabbit Model of Peritoneal Metastases: PIPALIM Project
Abstract
Background: Animal models are essential for testing new pressurized intraperitoneal aerosol chemotherapy (PIPAC) protocols; however, no immunocompetent animal model of peritoneal surface malignancies (PSMs) treated with PIPAC has been established. This study evaluated the feasibility, safety, and oncological efficacy of PIPAC in a rabbit PSM model.
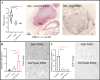
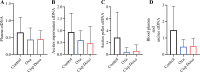
Methods: The study was conducted in two phases: (1) Feasibility Assessment: Three healthy rabbits underwent three consecutive PIPAC procedures (saline) at weekly intervals. The rabbits' well-being, morbidity, mortality, and histological changes were monitored. (2) Treatment Phase: Rabbits with PSM were treated with PIPAC using oxaliplatin, cisplatin-doxorubicin, or saline. Parameters such as animal well-being, ascites volume, morbidity, Peritoneal Cancer Index (PCI), histological response (Peritoneal Regression Grading Score [PRGS]), tumor cell proliferation/apoptosis, and circulating tumor DNA (ctDNA) levels were assessed.
Results: PIPAC was feasible and safe, with no increased morbidity or mortality. PIPAC demonstrated antitumor efficacy with lower PCI (control 21.6 vs. oxaliplatin 9.2 vs. cisplatin-doxorubicin 10.2; p < 0.001), improved histological response (PRGS: control 3.38 vs. oxaliplatin 1.95 vs. cisplatin-doxorubicin 1.85; p = 0.01), and reduced tumor cell proliferation (control 5.3% vs. oxaliplatin 0.82% vs. cisplatin-doxorubicin 0.62%; p < 0.0001). ctDNA levels showed promise for monitoring treatment response, warranting further investigation.
Conclusion: This study confirms the feasibility and effectiveness of PIPAC with oxaliplatin or cisplatin-doxorubicin in rabbits with PSM. The model provides a foundation for future research on PIPAC protocols and related treatments.
Keywords: Cancer; Intraperitoneal chemotherapy; PIPAC; Peritoneal metastases; Rabbit model.
© 2025. The Author(s).
Figures


References
-
- Taibi A, Teixeira Farinha H, Durand Fontanier S, Sayedalamin Z, Hübner M, Sgarbura O. Pressurized intraperitoneal aerosol chemotherapy enhanced by electrostatic precipitation (ePIPAC) for patients with peritoneal metastases. Ann Surg Oncol. 2021;28(7):3852–60. - PubMed
-
- Taliento C, Restaino S, Scutiero G, Arcieri M, Bernardi G, Martinello R, et al. Pressurized intraperitoneal aerosol chemotherapy (PIPAC) with cisplatin and doxorubicin in patients with ovarian cancer: a systematic review. Eur J Surg Oncol. 2023;49(12):107250. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

