Canadian Consensus Guidelines for the Management of Atopic Dermatitis with Topical Therapies
- PMID: 40279086
- PMCID: PMC12092898
- DOI: 10.1007/s13555-025-01386-2
Canadian Consensus Guidelines for the Management of Atopic Dermatitis with Topical Therapies
Abstract
Introduction: Atopic dermatitis (AD) is a highly prevalent disease in Canada with significant patient burden. Treatment guidance for topical therapy (the mainstay of AD management), with particular consideration of emerging treatments, may further improve patient care. Here, we aim to provide healthcare professionals with AD treatment recommendations from the perspective of 10 Canadian dermatologists with expertise in managing AD.
Methods: The panel of dermatologists conducted a systematic literature review and leveraged their clinical experience to develop generally accepted principles, consensus statements, and a treatment algorithm using an iterative consensus process.
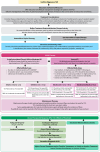
Results: The panel collectively developed six generally accepted principles, 10 consensus statements, and a treatment algorithm. The guidance notes that assessment of disease severity should encompass both physician-rated measures and patient-reported outcomes. Disease education, lifestyle-based strategies (e.g., trigger avoidance), and supportive measures (e.g., moisturizers) can help reduce signs and symptoms of AD. Choice of therapy should consider disease-, patient-, and treatment-related factors. Although topical corticosteroids (TCS) are often used as first-line treatment in AD, they should be limited to intermittent short-term use. Noncorticosteroid topical therapies (e.g., topical calcineurin inhibitors; topical phosphodiesterase-4 inhibitors; and topical Janus kinase inhibitors) can be used for widespread involvement of AD according to approved use. Once treatment goals are achieved, noncorticosteroid topical maintenance therapy should continue to prevent flares and reduce the need for TCS.
Conclusion: Guidance reflecting the benefits and limitations of topical AD treatments in conjunction with patient understanding of treatment goals supports robust shared decision-making in the management of AD.
Keywords: Atopic dermatitis; Janus kinase inhibitor; Phosphodiesterase-4 inhibitor; Topical calcineurin inhibitor; Topical corticosteroid.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of Interest: Melinda J. Gooderham has served as a principal investigator for AbbVie, Alumis, Amgen, AnaptysBio, Apogee, Arcutis, Aristea, Aslan, Bausch Health, Boehringer Ingelheim International GmbH, Bristol Myers Squibb, Cara Therapeutics, Coherus Biosciences, Dermira, Eli Lilly, Galderma SA, GlaxoSmithKline, Incyte Corporation, InMagene, JAMP, Janssen, LEO Pharma, MedImmune, Meiji, MoonLake, Nektar Therapeutics, Nimbus, Novartis, Pfizer, Regeneron, Sanofi Genzyme, Sun Pharma, Takeda Pharmaceutical Company, Tarsus, UCB, Ventyx, and Vyne; a consultant for AbbVie, Amgen, Apogee, Aslan, Bausch Health, Boehringer Ingelheim International GmbH, Eli Lilly, Janssen, Novartis Pharmaceuticals, Sanofi Genzyme, Sun Pharma, and UCB; an advisory board member for AbbVie, Amgen, Apogee, Arena Pharmaceuticals, Asana BioSciences, Aslan, Bausch Health, Boehringer Ingelheim International GmbH, Eli Lilly, Galderma SA, Incyte Corporation, Janssen, LEO Pharma, Novartis, Pfizer, Regeneron, Sanofi Genzyme, Sun Pharma, UCB, and Union; and a paid speaker for AbbVie, Amgen, Bausch Health, Bristol Myers Squibb, Boehringer Ingelheim International GmbH, Eli Lilly, Galderma SA, Janssen, JAMP, LEO Pharma, L’Oréal, Novartis, Pfizer, Regeneron, Sanofi Genzyme, Sun Pharma, and UCB. H. Chih-ho Hong has served as a speaker, advisor, consultant, and/or investigator for AbbVie, Amgen, Arcutis, Bausch Health, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Cutanea, Dermira, Dermavant, DS Biopharma, Eli Lilly, Galderma, GlaxoSmithKline, Incyte Corporation, Janssen, LEO Pharma, Medimmune, Merck, Mirimar, Novartis, Pfizer, Regeneron, Sanofi-Genzyme, Roche, and UCB. Charles Lynde has served as a speaker and/or consultant for AbbVie, Amgen, Aralez, Arcutis, Bausch Health, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Cipher, Dermavant, Eli Lilly, Fresnius Kabi, Galderma, GlaxoSmithKline, Incyte Corporation, Innovaderm, Intega Skin, Janssen, Kyowa Kirin, La Roche Posay, LEO Pharma, L’Oréal, Medexus, MedX, Merck, Novartis, P&G, Pediapharm, Pfizer, Regeneron, Roche, Sanofi Genzyme, Sandoz, Sentrex, Sun Pharma, TEVA, Tribute, UCB, Valeant, Viatris, and Volo Health and has served as a principal investigator for AbbVie, Acelyrin, Akros, Altius, Amgen, Aralez, Arcutis, Avillion, Bausch Health, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Celltrion, Cipher, Concert, Dermavant, Devonian, Eli Lilly, Evelo, Galderma, GlaxoSmithKline, Incyte Corporation, Innovaderm, Intega Skin, Janssen, Kyowa Kirin, La Roche Posay, LEO Pharma, L’Oréal, Medexus, MedX, Merck, MoonLake, Novartis, P&G, Pediapharm, Pfizer, Regeneron, Roche, Sanofi Genzyme, Sandoz, Sentrex, Sun Pharma, TEVA, Tribute, UCB, Valeant, Viatris, and Volo Health. Kim A. Papp has received honoraria and/or grants as a consultant, speaker, investigator, or scientific officer from AbbVie, Acelyrin, Akros, Alumis, Amgen, Arcutis, Bausch Health/Valeant, Boehringer Ingelheim, Bristol Myers Squibb, Can-Fite Biopharma, Celltrion, Concert Pharmaceuticals, Dermavant, Dermira, DICE Pharmaceuticals, DICE Therapeutics, Eli Lilly and Company, Evelo Biosciences, Forbion, Galderma, Horizon Therapeutics, Incyte Corporation, Janssen, Kymab, Kyowa Hakko Kirin, LEO Pharma, Meiji Seika Pharma, Mitsubishi Pharma, Nimbus Therapeutics, Novartis, Pfizer, Reistone, Sanofi-Aventis/Genzyme, Sandoz, Sun Pharma, Takeda, Tarsus Pharmaceuticals, UCB Pharma, and Zai Lab. Jensen Yeung has served as a consultant, investigator, or speaker or received honoraria from AbbVie, Amgen, Arcutis, Apogee, Bausch Health, Boehringer Ingelheim, Bristol Myers Squibb, Celltrion, Fresenius Kabi, Galderma, Incyte Corporation, JAMP Pharma, Janssen, LEO Pharma, Lilly, Novartis, Pfizer, Regeneron, Sanofi Genzyme, Sun Pharma, Takeda, and UCB. Harvey Lui has served as an advisor, consultant, investigator, and/or speaker for AbbVie, Incyte Corporation, L’Oréal, Novartis, and Vita Imaging. Yvette Miller-Monthrope has served as an advisor, consultant, and/or speaker for AbbVie, Bristol Myers Squibb, Boehringer Ingelheim, Fresenius Kabi, Galderma, Incyte Corporation, Janssen, Sanofi, Sun Pharma, Novartis, and UCB. Julien Ringuet has served as an advisor, consultant, and/or speaker for AbbVie, Amgen, Apogee, Arcutis, Bausch Health, Boehringer Ingelheim, Bristol Myers Squibb, Eli Lilly, Galderma, Incyte Corporation, Janssen, LEO Pharma, L’Oréal, NKS Health, Novartis, Organon, Pfizer, Sandoz, Sanofi Genzyme, Sun Pharma, and UCB and served as an investigator for AbbVie, Alumis, Amgen, Aristea, Aslan, Bristol Myers Squibb, Celgene, Concert Pharmaceuticals, CorEvitas, DICE Therapeutics, Incyte Corporation, Innovaderm, Janssen, LEO Pharma, Merck, Pfizer, Sanofi-Genzyme, Sun Pharma, and UCB. Irina Turchin has served as a speaker, advisor, consultant, or investigator for AbbVie, Amgen, Arcutis, Aristea, Bausch Health, Bristol Myers Squibb, Boehringer Ingelheim, Eli Lilly, Galderma, Horizon Therapeutics, Incyte Corporation, Janssen, Kiniksa, LEO Pharma, Mallinckrodt, MoonLake, Novartis, Pfizer, Sanofi, Sun Pharma, UCB, and Ventyx Biosciences. Vimal H. Prajapati has served as an advisor, consultant, and/or speaker for AbbVie, Actelion, Amgen, Apogee Therapeutics, Aralez, Arcutis, Aspen, Bausch Health, BioScript Solutions, Boehringer Ingelheim, Bristol Myers Squibb, Canadian Psoriasis Network, Celgene, Celltrion, Cipher, Concert, CorEvitas, Eczema Society of Canada, Eli Lilly, Galderma, GlaxoSmithKline, Homeocan, Incyte Corporation, JAMP Pharma, Janssen, Johnson & Johnson, LEO Pharma, Medexus, Novartis, Organon, Pediapharm, Pfizer, Sanofi Genzyme, Sun Pharma, Tribute, UCB, and Valeant; served as an investigator for AbbVie, AnaptysBio, Arcutis, Arena, Asana, Bausch Health, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Concert, CorEvitas, Dermavant, Dermira, Eli Lilly, Galderma, Incyte Corporation, Janssen, LEO Pharma, Meiji Pharma, Nektar Therapeutics, Nimbus Lakshmi, Novartis, Pfizer, RAPT Therapeutics, Regeneron, Reistone, Sanofi Genzyme, Sun Pharma, Takeda, and UCB; and received grants from AbbVie, Bausch Health, Janssen, LEO Pharma, Novartis, and Sanofi Genzyme. Ethical Approval: This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Figures
References
-
- Kirchhof MG, Landells I, Lynde CW, Gooderham MJ, Hong CH. Approach to the assessment and management of adult patients with atopic dermatitis: a consensus document. Section I: pathophysiology of atopic dermatitis and implications for systemic therapy. J Cutan Med Surg. 2018;22(1 suppl):6S-9S. - PubMed
-
- Kay J, Gawkrodger DJ, Mortimer MJ, Jaron AG. The prevalence of childhood atopic eczema in a general population. J Am Acad Dermatol. 1994;30(1):35–9. - PubMed
-
- Lee HH, Patel KR, Singam V, Rastogi S, Silverberg JI. A systematic review and meta-analysis of the prevalence and phenotype of adult-onset atopic dermatitis. J Am Acad Dermatol. 2019;80(6):1526–32. - PubMed
-
- Silverberg JI, Gelfand JM, Margolis DJ, et al. Patient burden and quality of life in atopic dermatitis in US adults: a population-based cross-sectional study. Ann Allergy Asthma Immunol. 2018;121(3):340–7. - PubMed
LinkOut - more resources
Full Text Sources

