Cumulative Burden of Digital Health Technologies for Patients With Multimorbidity: A Systematic Review
- PMID: 40279126
- PMCID: PMC12032558
- DOI: 10.1001/jamanetworkopen.2025.7288
Cumulative Burden of Digital Health Technologies for Patients With Multimorbidity: A Systematic Review
Abstract
Importance: Digital health technologies (DHTs) aiming to monitor, treat, and manage diseases can be prescribed for patients with multimorbidity; yet most DHTs are designed for individual conditions or problems, while approximately half of patients with chronic conditions have multiple chronic conditions.
Objectives: To identify DHTs approved by the US Food and Drug Administration (FDA) or listed in the Organisation for the Review of Care and Health Apps (ORCHA) library and prescribable for a hypothetical patient with 5 conditions and to model the number of DHTs this patient should be prescribed to receive benefits health professionals considered important.
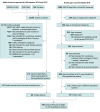
Evidence review: The FDA databases (Premarket Notification 510(k), Premarket Approval, and De Novo) and the ORCHA App Library from National Health Service Somerset were systematically searched for DHTs registered or updated between January 1, 2019, and December 31, 2022, that could be prescribed to a hypothetical woman with 5 chronic conditions (type 2 diabetes, hypertension, chronic obstructive pulmonary disease, osteoporosis, and osteoarthritis). After abstracting each DHT's elementary functions (ie, simple and delineated features to monitor, treat, and/or manage conditions), an assessment was undertaken to determine the fewest DHTs this hypothetical patient should be prescribed to receive benefit from digital functions health professionals considered important.
Findings: A total of 148 DHTs were identified (68 [46%] from FDA databases), of which 96 (65%) involved devices and 52 (35%) were standalone health apps. Only 5 DHTs (3.4%) were intended for 2 or more conditions. DHTs offered 140 elementary functions, ranging from recording, tracking, or visualizing health parameters to providing information to digital therapeutics with just-in-time interventions. The hypothetical patient would need to be prescribed up to 13 apps and 7 devices (a blood pressure monitor, a smartwatch, a pulse oximeter, a connected weight scale, a sensor-attached inhaler to monitor adherence, a lung function monitor, and a blood glucose sensor) to receive benefits from 28 functions at least 3 of 5 health professionals considered important.
Conclusions and relevance: This systematic review found that almost all prescribable DHTs were developed for a single condition or problem. Thus, patients with multiple chronic conditions would have to routinize many DHTs concurrently in daily life to benefit from digital functions health professionals considered important.
Conflict of interest statement
Figures

References
-
- US Food & Drug Administration . What is digital health? Accessed May 30, 2024. https://www.fda.gov/medical-devices/digital-health-center-excellence/wha...
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

