Brain feature maps reveal progressive animal-feature representations in the ventral stream
- PMID: 40279412
- PMCID: PMC12024516
- DOI: 10.1126/sciadv.adq7342
Brain feature maps reveal progressive animal-feature representations in the ventral stream
Abstract
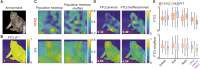
What are the fundamental principles that inform representation in the primate visual brain? While objects have become an intuitive framework for studying neurons in many parts of cortex, it is possible that neurons follow a more expressive organizational principle, such as encoding generic features present across textures, places, and objects. In this study, we used multielectrode arrays to record from neurons in the early (V1/V2), middle (V4), and later [posterior inferotemporal (PIT) cortex] areas across the visual hierarchy, estimating each neuron's local operation across natural scene via "heatmaps." We found that, while populations of neurons with foveal receptive fields across V1/V2, V4, and PIT responded over the full scene, they focused on salient subregions within object outlines. Notably, neurons preferentially encoded animal features rather than general objects, with this trend strengthening along the visual hierarchy. These results show that the monkey ventral stream is partially organized to encode local animal features over objects, even as early as primary visual cortex.
Figures





References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

