The P2X7R/NLRP3 inflammasome axis suppresses enthesis regeneration through inflammatory and metabolic macrophage-stem cell cross-talk
- PMID: 40279432
- PMCID: PMC12024643
- DOI: 10.1126/sciadv.adr4894
The P2X7R/NLRP3 inflammasome axis suppresses enthesis regeneration through inflammatory and metabolic macrophage-stem cell cross-talk
Abstract
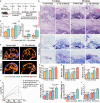
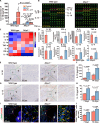
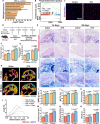
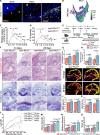
The regeneration of the enthesis remains a formidable challenge in regenerative medicine. However, key regulators underlying unsatisfactory regeneration remain poorly understood. This study reveals that the purinergic receptor P2X7 (P2X7R)/Nod-like receptor family protein 3 (NLRP3) inflammasome axis suppresses enthesis regeneration by amplifying IL-1β-mediated inflammatory cross-talk and suppressing docosatrienoic acid (DTA) metabolic cross-talk. NLRP3 inflammasomes were activated in macrophages following enthesis injury, thereby impairing the histological and functional recovery of the injured enthesis. Single-cell RNA sequencing (scRNA-seq) indicated that Nlrp3 knockout attenuated pathological inflammation and ameliorated the detrimental effects of IL-1β signaling cross-talk. Furthermore, NLRP3 inflammasomes suppressed the secretion of anti-inflammatory cytokines (IL-10 and IL-13) and DTA. The NLRP3 inflammasome-mediated secretome reduced differentiation and migration of stem cells. Neutralizing IL-1β or replenishing docosatrienoic acid accelerated enthesis regeneration. Moreover, conditional knockout of P2rx7 in myeloid cells attenuated NLRP3 inflammasome activation and facilitated enthesis regeneration. This study demonstrates that the P2X7R/NLRP3 inflammasome axis represents a promising therapeutic target for enthesis repair.
Figures





Similar articles
-
Aloe vera downregulates LPS-induced inflammatory cytokine production and expression of NLRP3 inflammasome in human macrophages.Mol Immunol. 2013 Dec;56(4):471-9. doi: 10.1016/j.molimm.2013.05.005. Epub 2013 Aug 1. Mol Immunol. 2013. PMID: 23911403
-
Potentiation of hepatic stellate cell activation by extracellular ATP is dependent on P2X7R-mediated NLRP3 inflammasome activation.Pharmacol Res. 2017 Mar;117:82-93. doi: 10.1016/j.phrs.2016.11.040. Epub 2016 Dec 8. Pharmacol Res. 2017. PMID: 27940204
-
P2X7 receptor inhibition attenuated sympathetic nerve sprouting after myocardial infarction via the NLRP3/IL-1β pathway.J Cell Mol Med. 2017 Nov;21(11):2695-2710. doi: 10.1111/jcmm.13185. Epub 2017 May 4. J Cell Mol Med. 2017. PMID: 28470940 Free PMC article.
-
P2X7 receptor antagonism prevents IL-1β release from salivary epithelial cells and reduces inflammation in a mouse model of autoimmune exocrinopathy.J Biol Chem. 2017 Oct 6;292(40):16626-16637. doi: 10.1074/jbc.M117.790741. Epub 2017 Aug 10. J Biol Chem. 2017. PMID: 28798231 Free PMC article.
-
The purinergic 2X7 receptor participates in renal inflammation and injury induced by high-fat diet: possible role of NLRP3 inflammasome activation.J Pathol. 2013 Nov;231(3):342-53. doi: 10.1002/path.4237. Epub 2013 Sep 3. J Pathol. 2013. PMID: 23843215
Cited by
-
Stem Cell Therapy: A New Approach and Effective Treatment for Psoriasis.Dermatol Pract Concept. 2025 Jul 31;15(3):5333. doi: 10.5826/dpc.1503a5333. Dermatol Pract Concept. 2025. PMID: 40790445 Free PMC article. Review.
-
Holistic regulation of the diabetic osteo-microenvironment via NIR-II nanocarriers with dual NO/pH responsiveness for enhanced bone regeneration.Bioact Mater. 2025 Aug 4;53:754-772. doi: 10.1016/j.bioactmat.2025.07.025. eCollection 2025 Nov. Bioact Mater. 2025. PMID: 40860012 Free PMC article.
References
-
- Wang X., Lin J., Li Z., Ma Y., Zhang X., He Q., Wu Q., Yan Y., Wei W., Yao X., Li C., Li W., Xie S., Hu Y., Zhang S., Hong Y., Li X., Chen W., Duan W., Ouyang H., Identification of an ultrathin osteochondral interface tissue with specific nanostructure at the human knee joint. Nano Lett. 22, 2309–2319 (2022). - PubMed
-
- Rossetti L., Kuntz L. A., Kunold E., Schock J., Muller K. W., Grabmayr H., Stolberg-Stolberg J., Pfeiffer F., Sieber S. A., Burgkart R., Bausch A. R., The microstructure and micromechanics of the tendon-bone insertion. Nat. Mater. 16, 664–670 (2017). - PubMed
-
- Atesok K., Fu F. H., Wolf M. R., Ochi M., Jazrawi L. M., Doral M. N., Lubowitz J. H., Rodeo S. A., Augmentation of tendon-to-bone healing. J. Bone Joint Surg. Am. 96, 513–521 (2014). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

