Device-Related Complications in Transvenous Versus Subcutaneous Defibrillator Therapy During Long-Term Follow-Up: The PRAETORIAN-XL Trial
- PMID: 40279654
- PMCID: PMC12272918
- DOI: 10.1161/CIRCULATIONAHA.125.074576
Device-Related Complications in Transvenous Versus Subcutaneous Defibrillator Therapy During Long-Term Follow-Up: The PRAETORIAN-XL Trial
Abstract
Background: The PRAETORIAN trial (A Prospective, Randomized Comparison of Subcutaneous and Transvenous Implantable Cardioverter Defibrillator Therapy) investigated the efficacy and safety of the subcutaneous implantable cardioverter defibrillator (S-ICD) compared with a transvenous ICD (TV-ICD) and showed noninferiority of the S-ICD with regard to the composite end point of device-related complications and inappropriate shocks after 49.1 months. Complications associated with transvenous leads are expected to occur after longer follow-up. The PRAETORIAN-XL trial aims to investigate whether the S-ICD is superior to the TV-ICD with respect to device-related complications at 8-year follow-up.
Methods: The PRAETORIAN trial randomized patients with a class I or IIa indication for ICD therapy without the need for pacing to either S-ICD or TV-ICD among 39 centers in the United States and Europe between March 2011 and January 2017. The follow-up was extended after 49.1 months by an additional 4 years for the PRAETORIAN-XL trial. The primary end point was the composite of all device-related complications. Complications could be related or unrelated to the lead and minor or major, with major complications being those requiring an invasive intervention. End points were analyzed according to the modified intention-to-treat principle using a Fine-Gray subdistribution hazards model to account for competing risks. An as-treated analysis was performed using a Cox proportional hazards model with device type as time-dependent variable.
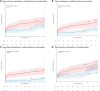
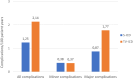
Results: Patients were randomized to S-ICD (n=426) and TV-ICD (n=423). Twenty-one percent of the S-ICD group versus 18% of the TV-ICD group were women. The median age at implantation was 63 (interquartile range, 54-69) years for the S-ICD and 64 (interquartile range, 56-69) years for the TV-ICD. After a median follow-up of 87.5 months, all device-related complications (major and minor combined) were not significantly different in the modified intention-to-treat analysis (subdistribution hazard ratio, 0.73 [95% CI, 0.48-1.12]); P=0.15). However, TV-ICD patients more often had a major complication or lead-related complication (P=0.03 and P<0.001, respectively). Moreover, the as-treated analysis showed significantly more complications in patients with a TV-ICD compared with an S-ICD (hazard ratio, 0.64 [95% CI, 0.41-0.99]; P=0.047).
Conclusions: The PRAETORIAN-XL trial demonstrated that there was no significant difference between the S-ICD and TV-ICD in all device-related complications during long-term follow-up. However, the TV-ICD carries a higher risk of major and lead-related complications compared with S-ICD therapy. The S-ICD should therefore be considered for all patients without a pacing indication who are evaluated for ICD therapy.
Registration: URL: https://www.clinicaltrials.gov; Unique identifier: NCT01296022.
Keywords: complications; defibrillators, implantable; electrophysiology.
Conflict of interest statement
R.E.K. reports consultancy fees and research grants from Abbott, Boston Scientific, Medtronic, and Cairdac and has stock options from AtaCor Medical Inc. M.F.E. reports consultancy fees from Boston Scientific and Medtronic. S.M. reports consultancy fees from Boston Scientific and Medtronic. K.M.K. reports consultancy fees from Boston Scientific. P.D.L. reports educational and research grants from and is on the research board of Boston Scientific and reports research grants from Abbott. K.V. reports consultancy fees from Medtronic and Abbott. M.C.B. is a consultant for and receives honoraria as well as research grants from Boston Scientific and has equity in and is chief medical officer for AtaCor Medical, Inc. D.J.W. has consultancy arrangements with Boston Scientific, Medtronic, and iRhythm and a research grant from Boston Scientific. P.N. reports modest speaker honoraria from Biotronik, Boston Scientific, and Medtronic. M.A.M. reports consultancy fees from Boston Scientific. Z.I.W. is an advisor for Boston Scientific and is on the advisory board for Medtronic and Abbot, and reports speaker fees from Medtronic.
Figures

References
-
- Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau R, Domanski M, Troutman C, Anderson J, Johnson G, et al. ; Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT) Investigators. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352:225–237. doi: 10.1056/NEJMoa043399 - PubMed
-
- Moss AJ, Hall WJ, Cannom DS, Daubert JP, Higgins SL, Klein H, Levine JH, Saksena S, Waldo AL, Wilber D, et al. Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. Multicenter automatic defibrillator implantation trial investigators. N Engl J Med. 1996;335:1933–1940. doi: 10.1056/NEJM199612263352601 - PubMed
-
- Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS, Daubert JP, Higgins SL, Brown MW, Andrews ML; Multicenter Automatic Defibrillator Implantation Trial II Investigators. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346:877–883. doi: 10.1056/NEJMoa013474 - PubMed
-
- Tarakji KG, Wazni OM, Harb S, Hsu A, Saliba W, Wilkoff BL. Risk factors for 1-year mortality among patients with cardiac implantable electronic device infection undergoing transvenous lead extraction: the impact of the infection type and the presence of vegetation on survival. Europace. 2014;16:1490–1495. doi: 10.1093/europace/euu147 - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical

