Hypoxia and loss of GCM1 expression prevent differentiation and contact inhibition in human trophoblast stem cells
- PMID: 40280139
- PMCID: PMC12143156
- DOI: 10.1016/j.stemcr.2025.102481
Hypoxia and loss of GCM1 expression prevent differentiation and contact inhibition in human trophoblast stem cells
Abstract
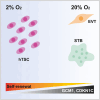
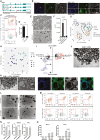
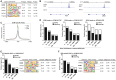
During the first stages of embryonic development, the placenta develops under very low oxygen tension (∼1%-2% O2), so we sought to determine the regulatory role of oxygen in human trophoblast stem cells (hTSCs). We find that low oxygen promotes hTSC self-renewal but inhibits differentiation to syncytiotrophoblast (STB) and extravillous trophoblast (EVT). The transcription factor GCM1 (glial cell missing transcription factor 1) is downregulated in low oxygen, and concordantly, there is substantial reduction of GCM1-regulated genes in hypoxic conditions. Knockout of GCM1 in hTSC likewise impaired EVT and STB formation. Treatment with a phosphatidylinositol 3-kinase (PI3K) inhibitor reported to reduce GCM1 protein levels likewise counteracts spontaneous or directed differentiation. Additionally, chromatin immunoprecipitation of GCM1 showed binding near key genes upregulated upon differentiation including the contact inhibition factor CDKN1C. Loss of GCM1 resulted in downregulation of CDKN1C and corresponding loss of contact inhibition, implicating GCM1 in regulation of this critical process.
Keywords: CDKN1C; GCM1; cell column; cytotrophoblast; differentiation; extravillous trophoblast; hypoxia; placenta; placental villi; syncytiotrophoblast; trophoblast stem cell.
Copyright © 2025 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures


Update of
-
Hypoxia and loss of GCM1 expression prevents differentiation and contact inhibition in human trophoblast stem cells.bioRxiv [Preprint]. 2024 Sep 10:2024.09.10.612343. doi: 10.1101/2024.09.10.612343. bioRxiv. 2024. Update in: Stem Cell Reports. 2025 May 13;20(5):102481. doi: 10.1016/j.stemcr.2025.102481. PMID: 39314437 Free PMC article. Updated. Preprint.
References
-
- Alsat E., Wyplosz P., Malassiné A., Guibourdenche J., Porquet D., Nessmann C., Evain-Brion D. Hypoxia impairs cell fusion and differentiation process in human cytotrophoblast, in vitro. J. Cell. Physiol. 1996;168:346–353. doi: 10.1002/(SICI)1097-4652(199608)168:2<346::AID-JCP13>3.0.CO;2-1. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

