In situ detection of activation of CAPN3, a responsible gene product for LGMDR1, in mouse skeletal myotubes
- PMID: 40280419
- PMCID: PMC12148437
- DOI: 10.1016/j.jbc.2025.108536
In situ detection of activation of CAPN3, a responsible gene product for LGMDR1, in mouse skeletal myotubes
Abstract
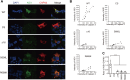
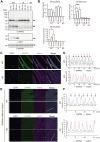
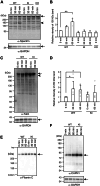
CAPN3/calpain-3/p94, a muscle-specific Ca2+-dependent cysteine protease, is responsible for limb-girdle muscular dystrophy R1 (LGMDR1), an autosomal recessive muscular dystrophy. However, the activation mechanism and physiological function of CAPN3 in skeletal muscles remain unknown. Here, we capture the in situ activation of CAPN3 in cultured mouse skeletal myotubes. Using our newly developed antibody, which specifically recognizes CAPN3 autolytic processing, we succeeded in differentiating WT CAPN3 from a protease-inactive CAPN3 mutant by immunostaining. We further demonstrated that CAPN3 predominantly localized at the M-bands of cultured skeletal myotubes at rest and translocated to the cytoplasm after activation by stimulation with ouabain, a cardiotonic steroid. This event requires a small but long-lasting cytoplasmic increase in Ca2+ levels, which is sufficient for the activation of CAPN3 but not of calpain-1/CAPN1. Activated CAPN3 digests the cytoskeletal proteins spectrin and talin. Thus, we successfully visualized the intracellular dynamics of endogenous CAPN3 in cultured skeletal muscles after activation by ouabain and demonstrated the subsequent processing of endogenous substrates in living cells. Our study will help understand the physiological functions of CAPN3 in skeletal muscles and the pathophysiological mechanisms of limb-girdle muscular dystrophy R1.
Keywords: calcium; calpain; muscular dystrophy; protein translocation; skeletal muscle.
Copyright © 2025 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures




References
-
- Richard I., Broux O., Allamand V., Fougerousse F., Chiannilkulchai N., Bourg N., et al. Mutations in the proteolytic enzyme calpain 3 cause limb-girdle muscular dystrophy type 2A. Cell. 1995;81:27–40. - PubMed
-
- Sorimachi H., Imajoh-Ohmi S., Emori Y., Kawasaki H., Ohno S., Minami Y., et al. Molecular cloning of a novel mammalian calcium-dependent protease distinct from both m- and μ-types. J. Biol. Chem. 1989;264:20106–20111. - PubMed
-
- Ohno S., Emori Y., Imajoh S., Kawasaki H., Kisaragi M., Suzuki K. Evolutionary origin of a calcium-dependent protease by fusion of genes for a thiol protease and a calcium-binding protein? Nature. 1984;312:566–570. - PubMed
-
- Emori Y., Kawasaki H., Sugihara H., Imajoh S., Kawashima S., Suzuki K. Isolation and sequence analyses of cDNA clones for the large subunits of two isozymes of rabbit calcium-dependent protease. J. Biol. Chem. 1986;261:9465–9471. - PubMed
-
- Campbell R.L., Davies P.L. Structure-function relationships in calpains. Biochem. J. 2012;447:335–351. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

