Allosteric communication mechanism in the glucagon receptor
- PMID: 40280422
- PMCID: PMC12145835
- DOI: 10.1016/j.jbc.2025.108530
Allosteric communication mechanism in the glucagon receptor
Abstract
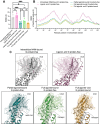
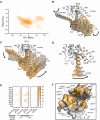
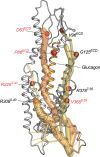
Recent drug development suggests agonists may be more promising against glucagon receptor dysregulation in metabolic disorders. Allosteric modulation may pave an alternative way to initiate responses that are required to target these metabolic disorders. Here, we investigated the allosteric communication mechanisms within the glucagon receptor using molecular dynamics simulations on five receptor states. Results highlighted that the extracellular domain is dynamic in the absence of an orthosteric agonist. In the presence of a partial agonist, we observed increased flexibility in the N terminus of the receptor compared with the full agonist-bound receptor. Class B1 G protein-coupled receptor (GPCR) microswitches showed repacking going from the inactive state to the active state, allowing for G protein coupling. In the full agonist- and G protein-bound state, Gαs showed both translational and rotational movement in the N terminus, core, and α5-helix, thereby forming key interactions between the core of the G protein and the receptor. Finally, the allosteric communication from the extracellular region to the G protein coupling region of the receptor was the strongest in the intracellular negative allosteric modulator-bound state, the full agonist and G protein-bound state, and the full agonist-bound G protein-free state. The residue positions predicted to play a significant role in the allosteric communication mechanism showed overlap with disease-associated mutations. Overall, our study provides insights into the allosteric communication mechanism in a class B1 GPCR, which sets the foundation for future design of allosteric modulators targeting the glucagon receptor.
Keywords: G protein; G protein–coupled receptor; SNP; allosteric communication; class B1; diabetes; glucagon; glucagon receptor; metabolic disorders; molecular dynamics.
Copyright © 2025 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures
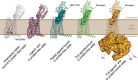



References
-
- Bromer W.W., Sinn L.G., Staub A., Behrens O.K. The amino acid sequence of glucagon. Diabetes. 1957;6:234–238. - PubMed
-
- Sutherland E.W., de Duve C. Origin and distribution of the hyperglycemic-glycogenolytic factor of the pancreas. J. Biol. Chem. 1948;175:663–674. - PubMed
-
- Cherrington A., Vranic M. Role of glucagon and insulin in control of glucose turnover. Metabolism. 1971;20:625–628. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

