Ashwagandha (Withania somnifera (L.) Dunal) for promoting recovery in long covid: protocol for a randomised placebo-controlled clinical trial (APRIL Trial)
- PMID: 40280611
- PMCID: PMC12035422
- DOI: 10.1136/bmjopen-2024-094526
Ashwagandha (Withania somnifera (L.) Dunal) for promoting recovery in long covid: protocol for a randomised placebo-controlled clinical trial (APRIL Trial)
Abstract
Background: Long covid describes a syndrome of persistent symptoms following COVID-19 and is responsible for substantial healthcare and economic burden. Currently, no effective treatments have been established. Ashwagandha (Withania somnifera (L.) Dunal) is a medicinal herb traditionally used in India for its immune-strengthening and anti-inflammatory properties. Withanolides, a family of steroid-derived molecules unique to Ashwagandha, have been shown to modulate inflammatory pathways in animal models, and several small randomised trials in humans support its effectiveness for reducing symptoms that are also associated with long covid. Therefore, this study aims to assess whether Ashwagandha is effective and safe for improving functional status and reducing symptom burden in adults living with long covid.
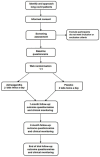
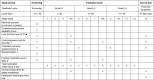
Methods: A randomised double-blind placebo-controlled trial will be performed at participating general practice (GP) surgeries and long covid clinics across the UK. Individuals diagnosed with long covid will be screened for eligibility and then randomised 1:1 to take 1000 mg daily of Ashwagandha root extract tablets (standardised to <0.9% withanolides) or matching placebo tablets for 3 months (target, n = 2500). Monthly online surveys will be performed to collect patient-reported outcomes, and monthly safety monitoring, including liver function tests, will be conducted by clinical site teams. The primary outcome of the Post-COVID Functional Status Scale score at 3 months will be assessed by baseline-adjusted ordinal logistic regression, according to a pre-published statistical analysis plan. The secondary outcomes included validated quality of life and long covid symptom scales, work status and productivity and adverse events. The trial has been approved as a Clinical Trial of an Investigational Medicinal Produce by the Medicines and Healthcare Regulatory Authority and by the NHS Research Ethics Committee and Health Research Authority.
Discussion: Treatments for long covid are urgently needed. This trial will robustly evaluate the safety and efficacy of a candidate treatment with a promising efficacy and safety profile. If found to be effective, the findings will likely influence treatment guidelines and improve health outcomes in those living with long covid.
Trial registration number: This trial was pre-registered on 15/08/2022: ISRCTN12368131.
Keywords: COMPLEMENTARY MEDICINE; Post-Acute COVID-19 Syndrome; Primary Care; Randomized Controlled Trial; Safety.
© World Health Organization 2025. Licensee BMJ.
Conflict of interest statement
Competing interests: Chief Investigator SK has no competing interests to declare. PM, MJ, MM, AP, TC, AS, RM, NB, AD, JL, SB, LH, AK, SAL, VK, SS and GKGP have no competing interests to declare. TN, DG and SR are employed by the All India Institute of Ayurveda which is an automonous research institute under the Ministry of AYUSH (the funder of this trial). AB is lead secretariat (voluntary role) for the All-Party Parliamentary Group on Indian Traditional Sciences, which aims to promote public understanding of Indian traditional sciences including Ayurveda in the UK and abroad. KK was chair of the ethnicity subgroup of the UK Scientific Advisory Group for Emergencies (SAGE) and is a member of SAGE. KK Chairs the National Long Covid Research Group which informs the Chief Medical Officer for England. Investigators at all clinical sites recruited to date have declared no competing interests.
Figures
References
-
- (ONS) O for NS . UK: 2023. Self-reported long covid symptoms.
-
- UK: ONS) O for NS; 2023. Prevalence of ongoing symptoms following coronavirus (covid-19) infection in the uk.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
