Heterogeneous Effects of Cognitive Arousal on the Contrast Response in Human Visual Cortex
- PMID: 40280712
- PMCID: PMC12178281
- DOI: 10.1523/JNEUROSCI.0798-24.2025
Heterogeneous Effects of Cognitive Arousal on the Contrast Response in Human Visual Cortex
Abstract
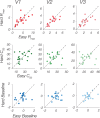
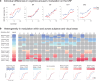
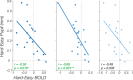
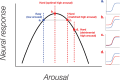
While animal studies have found that arousal states modulate visual responses, direct evidence for effects of arousal on human vision remains limited. Here, we used fMRI to examine effects of cognitive arousal on the gain of contrast response functions (CRFs) in human visual cortex. To measure CRFs, we measured BOLD responses in early visual cortex (V1-V3) while participants (n = 20, 14 females and 6 males) viewed stimuli that parametrically varied in contrast. To induce different cognitive arousal states, participants solved auditory arithmetic problems categorized as either Easy (low arousal) or Hard (high arousal). We found diversity in the modulatory effects across individuals: some individuals exhibited enhanced neural response with increased arousal, whereas others exhibited the opposite effect-a decrease in response with increased arousal. The pattern of overall BOLD modulation showed within-individual stability and was correlated with the degree of arousal-driven change in pupil size. Individuals who exhibited larger increases in pupil size with the arousal manipulation tended to show greater arousal-related decreases in visuocortical responses. We speculate that the polarity of the modulatory effect by cognitive arousal may relate to individual differences in cognitive effort expended in the high-difficulty condition, with individuals reaching different points on an underlying non-monotonic function.
Keywords: BOLD; arousal; contrast response functions; fMRI; vision.
Copyright © 2025 Pan et al.
Conflict of interest statement
The authors declare no competing financial interests.
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
