Investigating Mechanically Activated Currents from Trigeminal Neurons of Nonhuman Primates
- PMID: 40280765
- PMCID: PMC12071337
- DOI: 10.1523/ENEURO.0054-25.2025
Investigating Mechanically Activated Currents from Trigeminal Neurons of Nonhuman Primates
Abstract
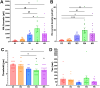
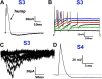
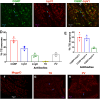
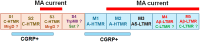
Pain sensation often involves mechanical modalities. Mechanically activated (MA) ion channels on sensory neurons underly responsiveness to mechanical stimuli. MA current properties have mainly been derived from rodent sensory neurons. This study aimed to address gaps in knowledge regarding MA current properties in trigeminal (TG) neurons of a higher-order species, common marmoset nonhuman primates (NHP). MA currents triggered by a piezoactuator were recorded in patch-clamp configuration. MA responses were associated with action potential (AP) properties, such as width, dV/dt on the falling phase, and presence/absence of AP firing in NHP TG neurons. According to responsiveness to mechanical stimuli and AP properties, marmoset TG neurons were clustered into four S-type and five M-type groups. S-type TG neurons had broader AP with two dV/dt peaks on the AP falling phase. Only one S-type group of NHP TG neurons produced small MA currents. M-type TG neurons had narrow AP without two dV/dt peaks on the AP falling phase. M-type NHP TG neurons, except for one group, showed MA currents. We additionally used immunohistochemistry to confirm the presence of known sensory neuronal types such as unmyelinated peptidergic CGRP+/trpV1+, unmyelinated nonpeptidergic MrgprD+ and CGRP-/trpV1+, and myelinated peptidergic CGRP+/trpV1- and nonpeptidergic CGRP- and PV+ NHP TG neurons. Overall, marmoset TG neurons and associated MA currents have many similarities compared with reported data from mouse sensory neurons. However, there are notable differences such as lower percentage of small NHP TG neurons responding to mechanical stimuli and absence of fast inactivating MA currents.
Keywords: mechnoactivated current; nonhuman primates; sensory neurons; trigeminal ganglia.
Copyright © 2025 Lindquist et al.
Conflict of interest statement
The authors declare no competing financial interests.
Figures




Update of
-
Investigating Mechanically Activated Currents from Trigeminal Neurons of Non-Human Primates.bioRxiv [Preprint]. 2024 Oct 7:2024.10.06.616876. doi: 10.1101/2024.10.06.616876. bioRxiv. 2024. Update in: eNeuro. 2025 May 12;12(5):ENEURO.0054-25.2025. doi: 10.1523/ENEURO.0054-25.2025. PMID: 39416195 Free PMC article. Updated. Preprint.
References
-
- Basbaum AI, Braz JM (2010) Transgenic mouse models for the tracing of “Pain” pathways.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
