Development and in vivo evaluation of a SARS-CoV-2 inactivated vaccine using high hydrostatic pressure
- PMID: 40280930
- PMCID: PMC12032236
- DOI: 10.1038/s41541-025-01136-7
Development and in vivo evaluation of a SARS-CoV-2 inactivated vaccine using high hydrostatic pressure
Abstract
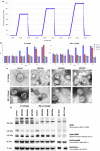
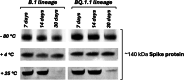
Developing low-cost vaccine production strategies is crucial to achieving global health equity and mitigating the spread and impact of disease outbreaks. High hydrostatic pressure (HHP) technology is a widely used technology employed in the food industry for long-term preservation. This project aims at validating HHP as a cost-effective method for the production of highly immunogenic thermal stable whole-virus SARS-CoV-2 vaccines. Structural studies on HHP-inactivated viruses demonstrated pressure-dependent effects, with higher pressures (500-600 MPa) destabilizing viral morphology. Immunogenicity assessments, in animal models, revealed that 500 MPa treatment elicited the most robust humoral and cellular immune responses, outperforming heat inactivation. Additionally, HHP-inactivated viral preparation retained thermostability for 30 days at 4 °C, reducing cold-chain dependencies and enabling vaccine distribution also in low-resource settings. With its rapid, cost-effective, and scalable production process, HHP presents a transformative, equitable solution for global vaccine development, particularly for emerging pathogens.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: There is a patent pending on the reported technology, number WO2023/248262A1. The inventors are P.R., Prof. V.S. and Dr. M.M. The remaining authors declare no competing interest.
Figures



References
-
- Thanh Le, T. et al. The COVID-19 vaccine development landscape. Nat. Rev. Drug Discov.19, 305–306 (2020). - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

