HER2DX ERBB2 mRNA score in first-line advanced HER2-positive breast cancer treated with chemotherapy, trastuzumab, and pertuzumab
- PMID: 40280938
- PMCID: PMC12032064
- DOI: 10.1038/s41523-025-00753-8
HER2DX ERBB2 mRNA score in first-line advanced HER2-positive breast cancer treated with chemotherapy, trastuzumab, and pertuzumab
Abstract
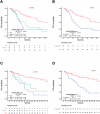
In advanced HER2-positive breast cancer, the standard taxane-trastuzumab-pertuzumab (THP) regimen faces competition from new therapies, emphasizing the need for biomarkers to guide treatment. This study evaluates the HER2DX ERBB2 mRNA score as a prognostic predictor, aiming to tailor treatment strategies. We retrospectively analyzed 94 patients treated with the THP regimen between 2010 and 2024. The HER2DX ERBB2 mRNA score was categorized as low (n = 14), medium (n = 20), or high (n = 60), and its correlation with progression-free survival (PFS) and overall survival (OS) was assessed using Cox regression models. The median follow-up was 31.5 months. Patients with ERBB2-high scores had significantly better median PFS (33.9 vs. 10.6 months, hazard ratio [HR] = 0.40, 95% CI: 0.24-0.69, p < 0.001) and OS (not reached vs. 30.8 months, HR = 0.26, 95% CI: 0.13-0.49, p < 0.001) compared to ERBB2-low patients. Based on these findings, further validation of this biomarker in tumor samples from the CLEOPATRA phase III trial is ongoing, which could help optimize treatment strategies in this population.
© 2025. The Author(s).
Conflict of interest statement
Ethics: The study was conducted in accordance with Good Clinical Practice guidelines and the World Medical Association Declaration of Helsinki. Patient anonymity was maintained by de-identifying clinical and genomic data before analysis. All patients provided written informed consent. The study protocol was approved by independent ethics committees at each participating institution (Institutional Review Board from Hospital Clinic Barcelona and Hospital Universitario 12 de Octubre). Competing interests: R.S.-B. reports advisory/consulting/speaker fees from Roche, AstraZeneca, Novartis, Lilly, Daiichi Sankyo, Pfizer, Eisai, GlaxoSmithKline, Reveal Genomics, and Gilead; travel expenses from Pfizer, AstraZeneca, Gilead, Novartis, and Roche. O.M.S. reports advisory/consulting fees from Reveal Genomics, Roche, and AstraZeneca, lecture fees from Daiichi Sankyo, Novartis, Pfizer, and Eisai, and travel expenses from Gilead and Novartis. E.S. declares personal fees for educational events and/or material from Novartis, Pfizer, Eisai, and Daiichi Sankyo; advisory fees from Pfizer and Seagen; and travel/accommodation expenses from Gilead, Daiichi Sankyo, Novartis, and Lilly. P.T. reports advisory and consulting fees from AstraZeneca, Daiichi-Sankyo, Adamed, Novartis, Pfizer, Lilly, Esteve, Gilead, Roche, and Reveal Genomics. T.P. reports advisory and consulting fees or speaker honoraria from Novartis, Astra Zeneca, Lilly, Pfizer, Veracyte, Gilead, and Roche, and support for attending meetings and/or travel from Gilead. A.M. reports advisory and consulting fees from AstraZeneca, Clovis (ended), GSK, MSD, PharmaMar, Pharma&, Immunogen. F.B-M. reports part-time employment from Reveal Genomics and has patents filed: PCT/EP2022/086493, PCT/EP2023/060810, EP23382703, and EP23383369. E.C. reports advisory/consulting/speaker fees from Roche, AstraZeneca, Novartis, Lilly, Daiichi Sankyo, Pfizer, and Gilead; travel expenses from Pfizer, AstraZeneca, MSD, Novartis, and Roche. A.P. reports advisory and consulting fees from AstraZeneca, Roche, Pfizer, Novartis, Daiichi Sankyo, Ona Therapeutics, and Peptomyc, lecture fees from AstraZeneca, Roche, Novartis, and Daiichi Sankyo, institutional financial interests from AstraZeneca, Novartis, Roche, and Daiichi Sankyo; stockholder and employee of Reveal Genomics; patents filed PCT/EP2016/080056, PCT/EP2022/086493, PCT/EP2023/060810, EP23382703, and EP23383369; editor of NPJ Breast Cancer journal. The remaining authors declare no competing interests.
Figures

References
-
- Swain, S. M. et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA): end-of-study results from a double-blind, randomised, placebo-controlled, phase 3 study. Lancet Oncol.21, 519–530 (2020). - PubMed
-
- Miles, D. et al. Final results from the PERUSE study of first-line pertuzumab plus trastuzumab plus a taxane for HER2-positive locally recurrent or metastatic breast cancer, with a multivariable approach to guide prognostication. Ann. Oncol.32, 1245–1255 (2021). - PubMed
-
- André, F. et al. Trastuzumab deruxtecan versus treatment of physician’s choice in patients with HER2-positive metastatic breast cancer (DESTINY-Breast02): a randomised, open-label, multicentre, phase 3 trial. Lancet401, 1773–1785 (2023). - PubMed
-
- Cortés, J. et al. Trastuzumab Deruxtecan versus Trastuzumab Emtansine for Breast Cancer. N. Engl. J. Med.386, 1143–1154 (2022). - PubMed
-
- Hurvitz, S. A. et al. Trastuzumab deruxtecan versus trastuzumab emtansine in patients with HER2-positive metastatic breast cancer: updated results from DESTINY-Breast03, a randomised, open-label, phase 3 trial. Lancet401, 105–117 (2023). - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

