Impact of single dose of pegfilgrastim on peripheral blood stem cell harvest in patients with multiple myeloma or malignant lymphoma
- PMID: 40281003
- PMCID: PMC12032086
- DOI: 10.1038/s41598-025-98453-7
Impact of single dose of pegfilgrastim on peripheral blood stem cell harvest in patients with multiple myeloma or malignant lymphoma
Abstract
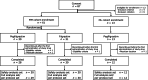
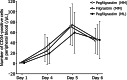
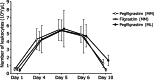
This phase 2 study evaluated the impact of pegfilgrastim, a single-dose, long-acting granulocyte colony-stimulating factor, on the steady-state mobilization of hematopoietic stem cells into peripheral blood in patients with multiple myeloma (MM) or malignant lymphoma (ML). Efficacy and safety, along with CD34-positive cell mobilization outcomes were assessed in patients with MM, who were randomly assigned to pegfilgrastim (n = 30) or daily filgrastim (n = 31), and ML (pegfilgrastim only, n = 13) cohorts. In the MM cohort, CD34-positive cell counts ≥ 2 × 106/kg were achieved in 100% of patients in the pegfilgrastim group and 96.7% in the filgrastim group (difference: 3.3%; 80% confidence interval: -0.9-7.5%), demonstrating the non-inferiority of pegfilgrastim to filgrastim. All patients in the ML cohort achieved ≥ 2 × 106/kg CD34-positive cell counts. The plerixafor administration rates in the MM cohort were 50.0% and 63.3% in the pegfilgrastim and filgrastim groups, respectively, and 91.7% in the ML cohort. There were no major differences in safety measures between the two groups. Although the sample size was small, particularly in the ML cohort, a single dose of pegfilgrastim demonstrated comparable efficacy and safety to daily doses of filgrastim, indicating its potential for clinical use while reducing patient burden.Trial Registration: jRCT2011210029, NCT05007652.
Keywords: CD34; Lymphoma; Mobilization; Multiple myeloma; Pegfilgrastim; Peripheral blood stem cell.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: Dr Goto has received all support for the present manuscript from Kyowa Kirin Co., Ltd.; research funding from Kyowa Kirin Co., Ltd., Sanofi K.K., Bristol-Myers Squibb K.K., and SymBio Pharmaceuticals Ltd.; lecture fees form Bristol-Myers Squibb K.K., Novartis Pharma K.K., Eisai Co., Ltd., SymBio Pharmaceuticals Ltd., MSD K.K., Kyowa Kirin Co., Ltd., Daiichi Sankyo Co., Ltd., Janssen Pharmaceutical K.K., Chugai Pharmaceutical Co., Ltd., AbbVie G.K., and Gilead Sciences, Inc.; and support for attending meetings and/or travel from Sanofi K.K. and Chugai Pharmaceutical Co., Ltd. Dr Sawa has received lecture fees from Kyowa Kirin Co., Ltd., Chugai Pharmaceutical Co., Ltd., Pfizer Japan Inc., Astellas Pharma Inc., Nippon Shinyaku Co., Ltd., Ono Pharmaceutical Co., Ltd., MSD K.K., Bristol-Myers Squibb K.K., Asahi Kasei Pharma Corporation, Novartis Pharma K.K., Eisai Co., Ltd., Otsuka Pharmaceutical Co., Ltd., Sumitomo Dainippon Pharma Co., Ltd., Sanofi K.K., Takeda Pharmaceutical Co., Ltd., Mundipharma K.K., AbbVie G.K., CSL Behring K.K., SymBio Pharmaceuticals Ltd., Janssen Pharmaceutical K.K., AstraZeneca K.K., Daiichi Sankyo Co., Ltd., Amgen K.K., Novo Nordisk Pharma Ltd., and Nippon Kayaku Co., Ltd.; and payment for expert testimony from Kyowa Kirin Co., Ltd., Takeda Pharmaceutical Co., Ltd., Daiichi Sankyo Co., Ltd., Asahi Kasei Pharma Corporation, and AbbVie G.K. Dr Fujiwara has received lecture fees from Asahi Kasei Pharma Corporation, Astellas Pharma Inc., Chugai Pharmaceutical Co., Ltd., CSL Behring K.K., Janssen Pharmaceutical K.K., Kyowa Kirin Co., Ltd., Merck Ltd., Novartis Pharma K.K., and Sanofi K.K. Dr Ishida has received research funding from Janssen Pharmaceutical K.K., Bristol-Myers Squibb K.K., Sanofi K.K., Alexion Pharmaceuticals, Inc., Pfizer Japan Inc., Takeda Pharmaceutical Co., Ltd., Kyowa Kirin Co., Ltd., and PRA Health Sciences, Inc.; and lecture fees from Ono Pharmaceutical Co., Ltd., Janssen Pharmaceutical K.K., Takeda Pharmaceutical Co., Ltd., CSL Behring K.K., Pfizer Japan Inc., Bristol-Myers Squibb K.K., and Sanofi K.K. Dr Ishitsuka has received research funding from Kyowa Kirin Co., Ltd., Ono Pharmaceutical Co., Ltd., AbbVie G.K., Genmab K.K., Daiichi Sankyo Co., Ltd., Pfizer Japan Inc., Bristol-Myers Squibb K.K., Mitsubishi Tanabe Pharma Corporation, Incyte Biosciences Japan G.K., Yakult Honsha Co., Ltd., and Eli Lilly Japan K.K.; consulting fees from Kyowa Kirin Co., Ltd., Daiichi Sankyo Co., Ltd., Meiji Seika Pharma Co., Ltd., and Yakult Honsha Co., Ltd.; and lecture fees from Kyowa Kirin Co., Ltd., Daiichi Sankyo Co., Ltd., Bristol-Myers Squibb K.K., Meiji Seika Pharma Co., Ltd., Chugai Pharmaceutical Co., Ltd., Ono Pharmaceutical Co., Ltd., AbbVie G.K., Janssen Pharmaceutical K.K., AstraZeneca K.K., Novartis Pharma K.K., Genmab K.K., Sawai Pharmaceutical Co., Ltd., Amgen K.K., Astellas Pharma Inc., Kissei Pharmaceutical Co., Ltd., Takeda Pharmaceutical Co., Ltd., and Sanofi K.K. Dr Toyosaki has received lecture fees from Janssen Pharmaceutical K.K., Chugai Pharmaceutical Co., Ltd., AstraZeneca K.K., Sanofi K.K., AbbVie G.K., Nippon Shinyaku Co., Ltd., Kyowa Kirin Co., Ltd., Asahi Kasei Pharma Corporation, Novartis Pharma K.K., Otsuka Pharmaceutical Co., Ltd., and Daiichi Sankyo Co., Ltd. Dr Sunami has received research funding from Ono Pharmaceutical Co., Ltd., Celgene K.K., AbbVie G.K., Sanofi K.K., Bristol-Myers Squibb K.K., GlaxoSmithKline K.K., Chugai Pharmaceutical Co., Ltd., Otsuka Pharmaceutical Co., Ltd., Janssen Pharmaceutical K.K., MSD K.K., Novartis Pharma K.K., Pfizer Japan Inc., Kyowa Kirin Co., Ltd., Incyte Biosciences Japan G.K., Mitsubishi Tanabe Pharma Corporation, and BeiGene, Ltd.; and lecture fees from Bristol-Myers Squibb K.K., Janssen Pharmaceutical K.K., and Sanofi K.K. Dr Sonoki has received research funding from Chugai Pharmaceutical Co., Ltd., Kyowa Kirin Co., Ltd., and Otsuka Pharmaceutical Co., Ltd.; and lecture fees from Alexion Pharmaceuticals, Inc., Otsuka Pharmaceutical Co., Ltd., Takeda Pharmaceutical Co., Ltd., Sanofi K.K., Novartis Pharma K.K., and Bristol-Myers Squibb K.K. Dr Miyamoto has received research funding from Kyowa Kirin Co., Ltd. and Chugai Pharmaceutical Co., Ltd.; and lecture fees from Takeda Pharmaceutical Co., Ltd., MSD K.K., Janssen Pharmaceutical K.K., Kyowa Kirin Co., Ltd., Otsuka Pharmaceutical Co., Ltd., Astellas Pharma Inc., and AbbVie G.K. Dr Hino has received research funding from Kyowa Kirin Co., Ltd.; consulting fees from Kyowa Kirin Co., Ltd.; and lecture fees from Kyowa Kirin Co., Ltd. Dr Maeda has received research funding from Asahi Kasei Pharma Corporation, Eisai Co., Ltd., Otsuka Pharmaceutical Co., Ltd., Kyowa Kirin Co., Ltd., Kyorin Pharmaceutical Co., Ltd., Taiho Pharmaceutical Co., Ltd., Takeda Pharmaceutical Co., Ltd., Chugai Pharmaceutical Co., Ltd., Teijin Pharma Ltd., Japan Blood Products Organization, Nippon Kayaku Co., Ltd., Nippon Shinyaku Co., Ltd., Mallinckrodt Pharma K.K., and Regimmune Co., Ltd.; lecture fees from Kyowa Kirin Co., Ltd., Astellas Pharma Inc., Amgen K.K., AbbVie G.K., Eisai Co., Ltd., Viatris Inc., Otsuka Pharmaceutical Co., Ltd., Ono Pharmaceutical Co., Ltd., Kyorin Pharmaceutical Co., Ltd., Kissei Pharmaceutical Co., Ltd., Gilead Sciences, Inc., Konica Minolta Inc., Sanofi K.K., JCR Pharmaceutical, Celgene K.K., Bristol-Myers Squibb K.K., CSL Behring K.K., Daiichi Sankyo Co., Ltd., Sumitomo Dainippon Pharma Co., Ltd., Takeda Pharmaceutical Co., Ltd., Terumo Corporation, Chugai Pharmaceutical Co., Ltd., Nippon Shinyaku Co., Ltd., Bayer Yakuhin, Ltd., Novartis Pharma K.K., Pfizer Japan Inc., PharmaEssentia Japan K.K., Mundipharma K.K., Human Life Cord Japan Inc., Meiji Seika Pharma Co., Ltd., Janssen Pharmaceutical K.K., and Yakult Honsha Co., Ltd.; and payment for expert testimony from Meiji Seika Pharma Co., Ltd., Medical Review Co., Ltd., and Asahi Kasei Pharma Corporation. Dr Teshima has received research funding from Chugai Pharmaceutical Co., Ltd., Fuji Pharma Co., Ltd., Nippon Shinyaku Co., Ltd., Nippon Kayaku Co., Ltd., Kyowa Kirin Co., Ltd., Eisai Co., Ltd., Sumitomo Pharma Co., Ltd., Asahi Kasei Pharma Corporation, Shionogi & Co., Ltd., JCR Pharmaceutical Co., Ltd., Astellas Pharma Inc., Otsuka Pharmaceutical Co., Ltd., Luca Science Inc., Priothera SAS, and PharmaEssentia Japan K.K.; consulting fees from Nippon Shinyaku Co., Ltd., Kyowa Kirin Co., Ltd., Meiji Seika Pharma Co., Ltd., Otsuka Pharmaceutical Co., Ltd., and Gilead Sciences, Inc.; lecture fees from Astellas Pharma Inc., AbbVie G.K., AstraZeneca K.K., Asahi Kasei Pharma Corporation, Gilead Sciences, Inc., Nippon Shinyaku Co., Ltd., Kyowa Kirin Co., Ltd., Meiji Seika Pharma Co., Ltd., Novartis Pharma K.K., Bristol-Myers Squibb K.K., Pfizer Japan Inc., Merck&Co., Inc., Otsuka Pharmaceutical Co., Ltd., Chugai Pharmaceutical Co., Ltd., Janssen Pharmaceutical K.K., Takeda Pharmaceutical Co., Ltd., and Nippon Kayaku Co., Ltd.; and was a director of the Japanese Society of Hematology; president of the Japanese Society for Transplantation and Cellular Therapy; and a director of the Japan Society of Transplantation Medicine and Cell Therapy. Mr/Ms Shimogomi, Ichihashi, and Ouchi are employees of Kyowa Kirin Co., Ltd. No other authors have a conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

