Alvespimycin is identified as a novel therapeutic agent for diabetic kidney disease by chemical screening targeting extracellular vesicles
- PMID: 40281012
- PMCID: PMC12032101
- DOI: 10.1038/s41598-025-98894-0
Alvespimycin is identified as a novel therapeutic agent for diabetic kidney disease by chemical screening targeting extracellular vesicles
Abstract
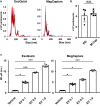
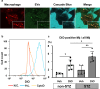
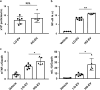
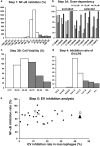
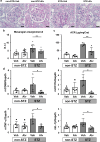
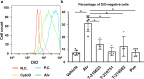
Extracellular vesicles (EVs) are important mediators of intercellular communication and play key roles in the regulation of pathophysiological processes. In diabetic kidney disease (DKD), it has been reported that macrophages recruited in the mesangial region may play pathogenic roles through inducing local inflammation in glomeruli. We focused on EV-mediated crosstalk between mesangial cells (MC) and macrophages as a novel therapeutic target for DKD. EVs released from MC induced inflammation in macrophages and the effect was enhanced under high-glucose conditions. For discovering novel therapeutic agents which can inhibit such EV-mediated mechanisms, drug repositioning is considered as an effective tool. We established a unique screening strategy and screened agents to aim at maximizing their specificity and potency to inhibit EV mechanisms, along with minimizing their toxicity. We succeeded in identifying alvespimycin, an HSP90 inhibitor. Treatment of diabetic rats with alvespimycin significantly suppressed mesangial expansion, inflammatory gene activation including macrophage markers, and proteinuria. The inhibitory effect on EV uptake was specific to alvespimycin compared with other known HSP90 inhibitors. MC-derived EVs are crucial for inflammation by intercellular crosstalk between MC and macrophages in DKD, and alvespimycin effectively ameliorated the progression of DKD by suppressing EV-mediated actions, suggesting that EV-targeted agents can be a novel therapeutic strategy.
Keywords: Diabetic kidney disease; Drug screening; Extracellular vesicle; Intraglomerular crosstalk; Macrophages; Mesangial cells.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures

Similar articles
-
Liraglutide ameliorates inflammation and fibrosis by downregulating the TLR4/MyD88/NF-κB pathway in diabetic kidney disease.Am J Physiol Regul Integr Comp Physiol. 2024 Oct 1;327(4):R410-R422. doi: 10.1152/ajpregu.00083.2024. Epub 2024 Aug 12. Am J Physiol Regul Integr Comp Physiol. 2024. PMID: 39133777 Free PMC article.
-
The anti-inflammation effect of Moutan Cortex on advanced glycation end products-induced rat mesangial cells dysfunction and High-glucose-fat diet and streptozotocin-induced diabetic nephropathy rats.J Ethnopharmacol. 2014;151(1):591-600. doi: 10.1016/j.jep.2013.11.015. Epub 2013 Nov 21. J Ethnopharmacol. 2014. PMID: 24269777
-
Suppressed ER-associated degradation by intraglomerular cross talk between mesangial cells and podocytes causes podocyte injury in diabetic kidney disease.FASEB J. 2020 Nov;34(11):15577-15590. doi: 10.1096/fj.202000078RR. Epub 2020 Sep 30. FASEB J. 2020. PMID: 32996639
-
Research progress on the role of extracellular vesicles in the pathogenesis of diabetic kidney disease.Ren Fail. 2024 Dec;46(1):2352629. doi: 10.1080/0886022X.2024.2352629. Epub 2024 May 20. Ren Fail. 2024. PMID: 38769599 Free PMC article. Review.
-
Extracellular vesicles and diabetic kidney disease: a systematic review.Eur Rev Med Pharmacol Sci. 2020 Sep;24(17):8978-8987. doi: 10.26355/eurrev_202009_22840. Eur Rev Med Pharmacol Sci. 2020. PMID: 32964987
References
-
- Tkach, M. & Thery, C. Communication by extracellular vesicles: Where we are and where we need to go. Cell164, 1226–1232 (2016). - PubMed
-
- Medeiros, T., Myette, R. L., Almeida, J. R., Silva, A. A. & Burger, D. Extracellular vesicles: Cell-derived biomarkers of glomerular and tubular injury. Cell Physiol. Biochem.54, 88–109 (2020). - PubMed
MeSH terms
Substances
Grants and funding
- 22K16223, 19K08729, 22K08311, 17K09706, 19K08728, 22K08357/Japan Society for the Promotion of Science
- 09021407/Strategic Grants from the Center for Metabolic Regulation of Healthy Aging, Kumamoto University Faculty of Life Sciences
- R4-6, R5-3/Kumamoto University Hospital Young Researcher Activation Project
- JP23ama121053/Platform Project for Supporting Drug Discovery and Life Science Research
LinkOut - more resources
Full Text Sources
Medical

