Soil-mimicking microfluidic devices reveal restricted flagellar motility of Bradyrhizobium diazoefficiens under microconfinement
- PMID: 40281045
- PMCID: PMC12032429
- DOI: 10.1038/s42003-025-07811-8
Soil-mimicking microfluidic devices reveal restricted flagellar motility of Bradyrhizobium diazoefficiens under microconfinement
Abstract
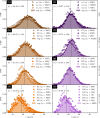
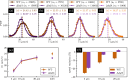
Bradyrhizobium diazoefficiens is a nitrogen-fixing symbiont of soybean, worldwide used as biofertilizer. This soil bacterium possesses two flagellar systems enabling it to swim in water-saturated soils. However, the motility in soil pores, which may be crucial for competitiveness for root nodulation, is difficult to predict. To address this gap, we fabricated microfluidic devices with networks of connected microchannels surrounding grains. In them, we directly visualise bacterial behaviour in transparent geometries mimicking minimalist soils-on-a-chip (SOCs). We measured the population velocities and changes of direction for two strains: the wild-type and a mutant with only a subpolar flagellum. A detailed statistical analysis revealed that both strains exhibited reduced speeds and increased changes of direction of 180°, in channels of decreasing cross sectional area, down to a few microns in width. Interestingly, while the wild-type strain displayed faster swimming in unconfined spaces, this advantage was negated in the SOCs with the narrowest microchannels. We employed the measured motility parameters to propose a realistic model and simulate B. diazoefficiens confined dynamics being able to reproduce their behaviour, which additionally can be extended enabling further predictions for long time and macro scales. This multidisciplinary work, combining design, microfabrication, microbiology and modelling, offers useful methods to study soil bacteria and may be readily extended to other beneficial/harmful soil species.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures





References
-
- Catroux, G., Hartmann, A. & Revellin, C. Trends in rhizobial inoculant production and use. Plant Soil230, 21–30 (2001).
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

