Cannabidiol exerts teratogenic effects on developing zebrafish through the sonic hedgehog signaling pathway
- PMID: 40281058
- PMCID: PMC12032106
- DOI: 10.1038/s41598-025-99194-3
Cannabidiol exerts teratogenic effects on developing zebrafish through the sonic hedgehog signaling pathway
Abstract
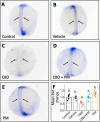
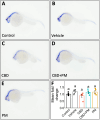
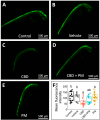
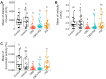
The increasing legalization and decriminalization of cannabis for therapeutic and recreational purposes in various regions have influenced public perceptions and attitudes toward cannabidiol (CBD)-containing products, including their use during pregnancy. However, there is a knowledge gap regarding the effect of CBD on fetal development, and the mechanisms by which CBD induces developmental deficits have not been well studied. In this study, we investigated whether the teratogenic effects of CBD on developing zebrafish are mediated through Sonic hedgehog signaling (Shh), a critical pathway in development. Embryonic exposure to CBD reduced hatching and survival rates and suppressed Shh pathway activity, leading to decreased ptch2 expression-a regulatory receptor expressed in response to Shh signaling. Larval swimming activity was impaired by CBD exposure. However, coexposure to CBD and a synthetic Shh pathway activator significantly improved developmental outcomes, including decreased mortality, increased hatching rates, elevated ptch2 expression, and increased locomotor activity. These findings underscore the developmental risks associated with CBD use during pregnancy and highlight the involvement of the Shh signaling pathway in driving these effects. The results of this study can inform regulations for cannabis use during pregnancy and emphasize the need to develop therapeutic guidelines for the safe use of CBD-based treatments.
Keywords: Cannabidiol; Early development; Gastrulation period; Sonic hedgehog signaling; Zebrafish.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures

Similar articles
-
Forebrain and hindbrain development in zebrafish is sensitive to ethanol exposure involving agrin, Fgf, and sonic hedgehog function.Birth Defects Res A Clin Mol Teratol. 2013 Jan;97(1):8-27. doi: 10.1002/bdra.23099. Epub 2012 Nov 27. Birth Defects Res A Clin Mol Teratol. 2013. PMID: 23184466 Free PMC article.
-
Multigenerational consequences of early-life cannabinoid exposure in zebrafish.Toxicol Appl Pharmacol. 2019 Feb 1;364:133-143. doi: 10.1016/j.taap.2018.12.021. Epub 2018 Dec 27. Toxicol Appl Pharmacol. 2019. PMID: 30594692 Free PMC article.
-
Deciphering the role of Shh signaling in axial defects produced by ethanol exposure.Birth Defects Res A Clin Mol Teratol. 2009 Jun;85(6):556-67. doi: 10.1002/bdra.20564. Birth Defects Res A Clin Mol Teratol. 2009. PMID: 19235835
-
Understanding dioxin developmental toxicity using the zebrafish model.Birth Defects Res A Clin Mol Teratol. 2006 Jan;76(1):7-18. doi: 10.1002/bdra.20216. Birth Defects Res A Clin Mol Teratol. 2006. PMID: 16333842 Review.
-
Chemical genetics: adding to the developmental biology toolbox.Dev Cell. 2003 Jul;5(1):11-9. doi: 10.1016/s1534-5807(03)00200-4. Dev Cell. 2003. PMID: 12852848 Review.
References
-
- Wheeldon, J. & Heidt, J. Cannabis criminology: Inequality, coercion, and illusions of reform. Drugs Educ. Prev. Policy29, 426–438 (2022).
-
- Lancione, S. et al. Non-medical cannabis in North America: An overview of regulatory approaches. Public Health178, 7–14 (2020). - PubMed
-
- Abuhasira, R., Shbiro, L. & Landschaft, Y. Medical use of cannabis and cannabinoids containing products–regulations in Europe and North America. Eur. J. Intern. Med.49, 2–6 (2018). - PubMed
-
- Imtiaz, S. et al. Cannabis legalization and cannabis use, daily cannabis use and cannabis-related problems among adults in Ontario, Canada (2001–2019). Drug Alcohol. Depend.244, 109765 (2023). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

