High-flow nasal oxygen vs. conventional oxygen therapy in patients with COVID-19 related acute hypoxemic respiratory failure and a do not intubate order: a multicentre cohort study
- PMID: 40281556
- PMCID: PMC12032817
- DOI: 10.1186/s12931-025-03231-8
High-flow nasal oxygen vs. conventional oxygen therapy in patients with COVID-19 related acute hypoxemic respiratory failure and a do not intubate order: a multicentre cohort study
Abstract
Background: High-flow nasal oxygen (HFNO) is frequently used to treat patients with acute hypoxemic respiratory failure (AHRF) due to viral pneumonia, including COVID-19. However, its clinical effect compared to conventional oxygen therapy (COT) remains largely unexplored in patients with a do not intubate (DNI) order. We aimed to assess whether HFNO compared to COT is associated with improved clinical outcomes in hospitalized patients with AHRF due to COVID-19 and a DNI order.
Methods: This analysis included patients with a DNI order and SARS-CoV-2 infection, selected from three observational studies, who were treated with COT only or HFNO. The primary endpoint was in-hospital mortality, the secondary endpoint was hospital length of stay (LOS). The effect of HFNO vs. COT was assessed using multivariable regression, accounting for pre-selected confounders.
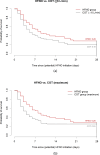
Results: Between March 2020 and September 2021, 116 patients received HFNO and 110 patients received COT. Median age was 78 [72-83], and 78% of the patients had a Clinical Frailty Scale score of 4 to 9. In-hospital mortality was 64% for HFNO and 71% for COT (p = 0.29), with an adjusted odds ratio of 0.72 (95% confidence interval [0.34-1.54], p = 0.40). Hospital LOS was 11 [6-18] days for HFNO, and 7 [4-12] days for COT (p < 0.001), with a remaining difference after adjusting for confounders (p < 0.01).
Conclusion: The lack of survival benefit and increased hospital LOS should be taken into account when considering HFNO for patients with a DNI order, suffering from AHRF due to viral pneumonia, like COVID-19.
Clinical trial registration: HFNO-COVID-19 study: DTR, NL9067 (Dutch Trial Registry), registration date: 27-11-2020.
Keywords: Acute hypoxemic respiratory failure; COVID-19; Conventional oxygen therapy; Do not intubate order; High-flow nasal oxygen.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The HFNO-COVID-19 cohort was approved by the local Medical Ethics Committee (MEC-U number W20.283). The Northwest Hospital group (NWZ) cohort was approved by the institutional research committee of the Northwest clinics (number L020 – 115). The University Medical Center Groningen (UMCG) cohort was approved by the local Medical Ethics Committee (METc number 2020/573). All studies have been carried out in accordance with the Declaration of Helsinki. Written informed consent was waived due to the observational character of the studies. However, some of the participating hospitals did obtain written informed consent if deemed necessary by local guidelines of institutional research committees. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

