Cost-effectiveness and public health impact of recombinant zoster vaccine versus no herpes zoster vaccination in selected populations of immunocompromised adults in Canada
- PMID: 40281614
- PMCID: PMC12023514
- DOI: 10.1186/s12913-025-12550-x
Cost-effectiveness and public health impact of recombinant zoster vaccine versus no herpes zoster vaccination in selected populations of immunocompromised adults in Canada
Abstract
Background: The risk of herpes zoster (HZ) increases with age and in immunocompromised (IC) patients. Recombinant zoster vaccine (RZV) is currently recommended in Canada for people aged ≥ 50 years. The objectives of the current study were to evaluate the cost-effectiveness and public health impact of RZV versus no HZ vaccination in select Canadian IC adult populations.
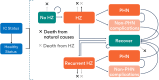
Methods: The ZOster ecoNomic Analysis ImmunoCompromised (ZONA IC) model followed a base-case cohort of 1600 patients with hematopoietic stem-cell transplant (HSCT) from a starting age of 55 years, who maintained IC status for 5 years, from a societal perspective. Scenario analyses were conducted for patients with breast cancer, renal transplant, human immunodeficiency virus (HIV), and Hodgkin lymphoma. These probabilistic analyses used a life-long time horizon and discount rates of 1.5% for costs and quality-adjusted life-years (QALYs). First-dose coverage was assumed to be 60% and second-dose completion 100%. Deterministic one-way sensitivity analysis for the base case was performed. Costs are reported in 2022 Canadian dollars, with an assumed cost-effectiveness threshold of $50,000 per QALY gained.
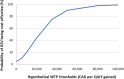
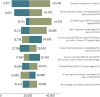
Results: In the base-case analysis (HSCT), it was estimated that RZV would prevent medians of 116 HZ and 27 postherpetic neuralgia (PHN) cases, respectively versus no HZ vaccination. Estimated median numbers needed to vaccinate were 8 and 35 to avoid one HZ and one PHN case, respectively. The median incremental cost-effectiveness ratio (ICER) was $22,648 per QALY gained and was most sensitive to assumptions of HZ incidence, direct medical costs for unvaccinated HZ without PHN, and RZV efficacy against PHN. In other IC populations, estimated median ICERs were $24,328 (breast cancer), $27,237 (renal transplant), $67,207 (HIV), and $81,470 (Hodgkin lymphoma).
Conclusions: RZV in Canada improves public health outcomes and is likely cost-effective for several IC conditions.
Keywords: Breast cancer; Canada; Hematopoietic stem-cell transplant; Hodgkin lymphoma; Human immunodeficiency virus; Immunocompromised; Recombinant zoster vaccine; Renal transplant.
© 2025. GSK.
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: DL, IK, CN, and DC are employed by, and holds financial equities in GSK. SG is employed by GSK. JC and KH are employed by RTI - Health Solutions and their employer received consulting fees from GSK to perform the work contributing to this research. All authors declare no other financial and non-financial relationships and activities.
Figures
Similar articles
-
Public Health Impact and Cost-Effectiveness of Non-live Adjuvanted Recombinant Zoster Vaccine in Canadian Adults.Appl Health Econ Health Policy. 2019 Oct;17(5):723-732. doi: 10.1007/s40258-019-00491-6. Appl Health Econ Health Policy. 2019. PMID: 31250218 Free PMC article.
-
Public health impact of recombinant zoster vaccine for prevention of herpes zoster in US adults immunocompromised due to cancer.Hum Vaccin Immunother. 2023 Dec 31;19(1):2167907. doi: 10.1080/21645515.2023.2167907. Epub 2023 Mar 7. Hum Vaccin Immunother. 2023. PMID: 36880669 Free PMC article.
-
Health economic evaluation of vaccination strategies for the prevention of herpes zoster and postherpetic neuralgia in Germany.BMC Health Serv Res. 2013 Sep 26;13:359. doi: 10.1186/1472-6963-13-359. BMC Health Serv Res. 2013. PMID: 24070414 Free PMC article.
-
Development of adjuvanted recombinant zoster vaccine and its implications for shingles prevention.Expert Rev Vaccines. 2018 Jul;17(7):619-634. doi: 10.1080/14760584.2018.1495565. Epub 2018 Jul 20. Expert Rev Vaccines. 2018. PMID: 30028651 Review.
-
Cost-effectiveness of vaccination against herpes zoster.Hum Vaccin Immunother. 2014;10(7):2048-61. doi: 10.4161/hv.28670. Hum Vaccin Immunother. 2014. PMID: 25424815 Free PMC article. Review.
References
-
- Government of Canada. Herpes zoster (shingles) vaccine: Canadian immunization guide. https://www.canada.ca/en/public-health/services/publications/healthy-liv.... Accessed 27 Apr 2023.
-
- Brisson M, Pellissier JM, Camden S, Quach C, De Wals P. The potential cost-effectiveness of vaccination against herpes zoster and post-herpetic neuralgia. Hum Vaccin. 2008;4(3):238–45. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

