Cardiac Fibroblasts: Helping or Hurting
- PMID: 40282342
- PMCID: PMC12026832
- DOI: 10.3390/genes16040381
Cardiac Fibroblasts: Helping or Hurting
Abstract
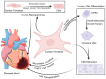
Cardiac fibroblasts (CFs) are the essential cell type for heart morphogenesis and homeostasis. In addition to maintaining the structural integrity of the heart tissue, muscle fibroblasts are involved in complex signaling cascades that regulate cardiomyocyte proliferation, migration, and maturation. While CFs serve as the primary source of extracellular matrix proteins (ECM), tissue repair, and paracrine signaling, they are also responsible for adverse pathological changes associated with cardiovascular disease. Following activation, fibroblasts produce excessive ECM components that ultimately lead to fibrosis and cardiac dysfunction. Decades of research have led to a much deeper understanding of the role of CFs in cardiogenesis. Recent studies using the single-cell genomic approach have focused on advancing the role of CFs in cellular interactions, and the mechanistic implications involved during cardiovascular development and disease. Arguably, the unique role of fibroblasts in development, tissue repair, and disease progression categorizes them into the friend or foe category. This brief review summarizes the current understanding of cardiac fibroblast biology and discusses the key findings in the context of development and pathophysiological conditions.
Keywords: cardiac fibrosis; cardiac muscle; cardiomyocytes; cardiomyopathy; fibroblasts; signaling.
Conflict of interest statement
The authors have no competing interests.
Figures



References
-
- Abercrombie M., Flint M.H., James D.W. Wound Contraction in Relation to Collagen Formation in Scorbutic Guinea-Pigs. J. Embryol. Exp. Morphol. 1956;4:167–175.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

