Transcriptome Analysis Reveals Key Genes Involved in Fatty Acid and Triacylglycerol Accumulation in Developing Sunflower Seeds
- PMID: 40282355
- PMCID: PMC12026707
- DOI: 10.3390/genes16040393
Transcriptome Analysis Reveals Key Genes Involved in Fatty Acid and Triacylglycerol Accumulation in Developing Sunflower Seeds
Abstract
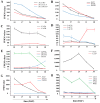
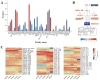
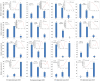
Background/objectives: Sunflower (Helianthus annuus L.) is one of the four major global oilseed crops. Understanding the molecular mechanisms regulating fatty acid synthesis and triacylglycerol (TAG) accumulation is crucial for improving oil yield and quality. In this study, the oilseed sunflower cultivar 'T302', which was wild-cultivated in the northwestern region of China, was analyzed for fatty acid content by targeted lipidomic analysis. RNA sequencing (RNA-seq) was performed on 15 cDNA libraries from sunflower embryos at five developmental stages (10, 17, 24, 31, and 38 days after flowering) to investigate gene expression patterns during oil accumulation. Differentially expressed genes (DEGs) related to fatty acid and triacylglycerol accumulation in developing sunflower seeds were identified. WGCNA was used to gain deeper insights into the mechanisms underlying lipid metabolism.
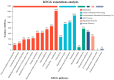
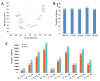
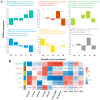
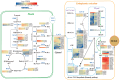
Results: The oil composition of 'T302' consisted of 86.61% unsaturated fatty acids (UFA), mainly linoleic acid (48.47%) and oleic acid (37.25%). Saturated fatty acids (SFAs) accounted for 13.39%, with palmitic acid (7.46%) and stearic acid (5.04%) being the most abundant. A total of 81,676 unigenes were generated from RNA-seq data, and 91 DEGs associated with lipid metabolism were identified, including key enzymes such as FAD2-1, SAD, FATA, LACS, PDAT2, and DGAT2. In addition, we identified several novel candidate transcription factor genes, including WRI1, LEC1, FUS3, and ABI3, which were found to regulate TAG synthesis during seed maturation and are worthy of further investigation. This study provides valuable insights into the molecular mechanisms of seed oil biosynthesis in oilseed sunflower. The identified key genes and transcription factors provide potential targets for molecular breeding strategies to increase oil content and modify fatty acid compositions in sunflower and other oilseed crops.
Keywords: developing seeds; lipid biosynthesis; sunflower (Helianthus annuus L.); transcriptome; unsaturated fatty acids.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Transcriptome Analysis Reveals Metabolic Pathways and Key Genes Involved in Oleic Acid Formation of Sunflower (Helianthus annuus L.).Int J Mol Sci. 2025 Jul 15;26(14):6757. doi: 10.3390/ijms26146757. Int J Mol Sci. 2025. PMID: 40725004 Free PMC article.
-
Transcriptomic analysis of Perilla frutescens seed to insight into the biosynthesis and metabolic of unsaturated fatty acids.BMC Genomics. 2018 Mar 21;19(1):213. doi: 10.1186/s12864-018-4595-z. BMC Genomics. 2018. PMID: 29562889 Free PMC article.
-
Identification of genes associated with the biosynthesis of unsaturated fatty acid and oil accumulation in herbaceous peony 'Hangshao' (Paeonia lactiflora 'Hangshao') seeds based on transcriptome analysis.BMC Genomics. 2021 Feb 1;22(1):94. doi: 10.1186/s12864-020-07339-7. BMC Genomics. 2021. PMID: 33522906 Free PMC article.
-
Genetic possibilities for altering sunflower oil quality to obtain novel oils.Can J Physiol Pharmacol. 2008 Apr;86(4):215-21. doi: 10.1139/Y08-008. Can J Physiol Pharmacol. 2008. PMID: 18418432 Review.
-
Regulation of Oil Biosynthesis and Genetic Improvement in Plants: Advances and Prospects.Genes (Basel). 2024 Aug 26;15(9):1125. doi: 10.3390/genes15091125. Genes (Basel). 2024. PMID: 39336716 Free PMC article. Review.
References
-
- Khan S., Choudhary S., Pandey A., Khan M.K., Thomas G. Sunflower oil: Efficient oil source for human consumption. Emerg. Life Sci. Res. 2015;1:1–3.
-
- Seiler G.J., Qi L.L., Marek L.F. Utilization of sunflower crop wild relatives for cultivated sunflower improvement. Crop Sci. 2017;57:1083–1101. doi: 10.2135/cropsci2016.10.0856. - DOI
-
- Flagella Z., Rotunno T., Tarantino E., Di Caterina R., De Caro A. Changes in seed yield and oil fatty acid composition of high oleic sunflower (Helianthus annuus L.) hybrids in relation to the sowing date and the water regime. Eur. J. Agron. 2002;17:221–230. doi: 10.1016/S1161-0301(02)00012-6. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

