SYNGAP1 Syndrome and the Brain Gene Registry
- PMID: 40282364
- PMCID: PMC12026819
- DOI: 10.3390/genes16040405
SYNGAP1 Syndrome and the Brain Gene Registry
Abstract
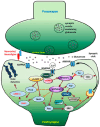
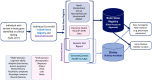
Background: The human brain relies on complex synaptic communication regulated by key genes such as SYNGAP1. SYNGAP1 encodes the GTPase-Activating Protein (SYNGAP), a critical synaptic plasticity and neuronal excitability regulator. Impaired SYNGAP1 function leads to neurodevelopmental disorders (NDDs) characterized by intellectual disability (ID), epilepsy, and behavioral abnormalities. These variants disrupt Ras signaling, altering AMPA receptor transport and synaptic plasticity and contributing to cognitive and motor difficulties. Despite advancements, challenges remain in defining genotype-phenotype correlations and distinguishing SYNGAP1-related disorders from other NDDs, which could improve underdiagnosis and misdiagnosis. Brain Gene Registry: The Brain Gene Registry (BGR) was established as a collaborative initiative, consolidating genomic and phenotypic data across multiple research centers. This database allows for extensive analyses, facilitating improved diagnostic accuracy, earlier interventions, and targeted therapeutic strategies. The BGR enhances our understanding of rare genetic conditions and is critical for advancing research on SYNGAP1-related disorders.
Conclusions: While no FDA-approved treatments exist for SYNGAP1-related disorders, several therapeutic approaches are being investigated. These include taurine supplementation, ketogenic diets, and molecular strategies such as antisense oligonucleotide therapy to restore SYNGAP1 expression. Behavioral and rehabilitative interventions remain key for managing developmental and cognitive symptoms. Advancing research through initiatives like the BGR is crucial for refining genotype-phenotype associations and developing precision medicine approaches. A comprehensive understanding of SYNGAP1-related disorders will improve clinical outcomes and patient care, underscoring the need for continued interdisciplinary collaboration in neurodevelopmental genetics.
Keywords: SYNGAP1; brain gene registry; intellectual disability; neurodevelopmental disorder.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures
Similar articles
-
SYNGAP1 mutations: Clinical, genetic, and pathophysiological features.Int J Dev Neurosci. 2019 Nov;78:65-76. doi: 10.1016/j.ijdevneu.2019.08.003. Epub 2019 Aug 24. Int J Dev Neurosci. 2019. PMID: 31454529 Review.
-
Comprehensive phenotypes of patients with SYNGAP1-related disorder reveals high rates of epilepsy and autism.Epilepsia. 2024 May;65(5):1428-1438. doi: 10.1111/epi.17913. Epub 2024 Mar 12. Epilepsia. 2024. PMID: 38470175 Free PMC article.
-
Species-conserved SYNGAP1 phenotypes associated with neurodevelopmental disorders.Mol Cell Neurosci. 2018 Sep;91:140-150. doi: 10.1016/j.mcn.2018.03.008. Epub 2018 Mar 24. Mol Cell Neurosci. 2018. PMID: 29580901 Free PMC article. Review.
-
Phenotypic characterization of individuals with SYNGAP1 pathogenic variants reveals a potential correlation between posterior dominant rhythm and developmental progression.J Neurodev Disord. 2019 Aug 8;11(1):18. doi: 10.1186/s11689-019-9276-y. J Neurodev Disord. 2019. PMID: 31395010 Free PMC article.
-
Behavioural and neurodevelopmental characteristics of SYNGAP1.J Neurodev Disord. 2024 Aug 15;16(1):46. doi: 10.1186/s11689-024-09563-8. J Neurodev Disord. 2024. PMID: 39148034 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

