The Interconnection Between UbcH10, p53, and EGFR in Lung Cancer Cells and Their Involvement in Treatment Response
- PMID: 40282365
- PMCID: PMC12026858
- DOI: 10.3390/genes16040404
The Interconnection Between UbcH10, p53, and EGFR in Lung Cancer Cells and Their Involvement in Treatment Response
Abstract
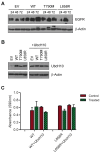
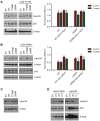
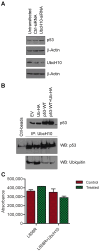
Background/Objectives: The UbcH10 protein plays an important role in a variety of human malignancies, including thyroid, breast, ovarian, and colorectal carcinomas. It has been previously reported that UbcH10 is overexpressed in non-small cell lung cancer (NSCLC) compared to normal lungs and that its expression is directly and inversely correlated with the mutational status of p53 and EGFR, respectively. Methods: We transfected lung cancer cells with wild-type and mutant forms of EGFR, modulated the expression of UbcH10 and p53, and treated these cells with tyrosine kinase inhibitor (TKI) erlotinib. Using Western blotting, we evaluated the expression of UbcH10 induced by EGFR and p53. Finally, we employed immunohistochemistry to assess the levels of UbcH10 expression in a subset of NSCLC patients receiving TKI therapy. Results: We reported a possible modulation of UbcH10 expression by the overexpression of wild-type and mutant EGFR in H460 lung cancer cells, potentially through p53. The enforced expression of UbcH10 in cells transfected with mutant EGFR suggested a potential increase in resistance to erlotinib treatment. Finally, immunohistochemical analysis of samples from NSCLC patients with mutant EGFR indicated a possible connection between UbcH10 expression levels and progression-free survival. Conclusions: In NSCLC, UbcH10 may play a role in the regulation of TKI response via a molecular pathway potentially involving p53 and EGFR. However, further research is needed to fully understand this mechanism.
Keywords: EGFR; UbcH10; adenocarcinoma; non-small cell lung cancer; p53; resistance.
Conflict of interest statement
U.M., Consulting or advisory role (unrelated to the current work): Boehringer Ingelheim, MSD, Roche, Amgen, Lilly, Thermo Fisher Scientific, Diaceutics, Merck, Glaxo Smith Kline, Astra Zeneca; Speakers’ Bureau (unrelated to the current work): Boehringer Ingelheim, Roche, AstraZeneca, MSD, Merck, Amgen, Thermo Fisher Scientific, Diaceutics, Lilly, Glaxo Smith Kline. F.P., Relationship unrelated to the current work (advisory fees, honoraria, grants, travel accommodation and expenses, and non-financial support) with Menarini and Roche. All other authors declare no conflicts of interest. The funder had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures

References
-
- Reck M., Remon J., Hellmann M.D. First-Line Immunotherapy for Non–Small-Cell Lung Cancer. J. Clin. Oncol. 2022;40:586–597. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

