Long-Term Oncological Outcomes of Granulocyte Colony-Stimulating Factor (G-CSF) Treatment in Gastrointestinal Cancers: A Systematic Review and Meta-Analysis
- PMID: 40282489
- PMCID: PMC12026166
- DOI: 10.3390/cancers17081313
Long-Term Oncological Outcomes of Granulocyte Colony-Stimulating Factor (G-CSF) Treatment in Gastrointestinal Cancers: A Systematic Review and Meta-Analysis
Abstract
Background: Granulocyte-colony stimulating factor (G-CSF) prophylaxis is widely used in gastrointestinal (GI) cancers. The use of G-CSF in GI cancers has not previously been investigated systematically in a meta-analysis. Thus, we systematically reviewed the literature to describe the G-CSF use and potential influence on long-term oncological outcomes in GI cancers.
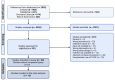
Method: The literature search of this systematic review and meta-analysis was conducted in PubMed, Embase, Cochrane Library and Web of Science. The PRISMA-P guidelines were followed. Studies that reported data on patients with GI cancers undergoing oncological treatment with G-CSF prophylaxis were included. Outcomes of interest were overall survival (OS), progression-free survival (PFS) and adverse events (AE), specifically neutropenia grade III/IV. A time-to-event random-effects meta-analysis was conducted. Risk of bias was assessed using the Newcastle-Ottawa Scale and the Cochrane Risk of Bias Tool for Randomized Controlled Trials (RoB) tool.
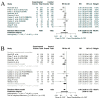
Findings: In total, 2452 articles were screened for eligibility. Ultimately, 13 studies were included with a total patient number of 2673. The included studies indicated a positive association between OS and G-CSF prophylaxis (HR 0.72, 95% CI: 0.56-0.91, I2: 54%, low quality evidence). No significant relation between G-CSF use and PFS was found in the pooled analyses (HR 0.74, 95% CI: 0.51-1.08, I2: 73%, moderate quality evidence). However, a positive effect of G-CSF use was found in the retrospective cohorts reporting data on PFS (HR 0.50, 95% CI: 0.32-0.77, I2: 0%). A marked drop in neutropenia grade III/IV rates was observed in patients treated with G-CSF (risk ratio (RR) 0.46, 95% CI: 0.28-0.77, I2: 72%, high quality evidence).
Interpretation: G-CSF prophylaxis provides a reduction in neutropenia grade III/IV in patients with GI cancers (high level of certainty) and a favorable OS (low certainty), while PFS is unaffected (moderate certainty). Studies on PFS and G-CSF use are nonetheless limited.
Keywords: G-CSF; adverse events; filgrastim; gastrointestinal cancer; peg-filgrastim; survival.
Conflict of interest statement
All the authors of this manuscript declare no conflicts of interest.
Figures


References
-
- Trotta F., Mayer F., Mecozzi A., Amato L., Addis A. Impact of Guidance on the Prescription Patterns of G-CSFs for the Prevention of Febrile Neutropenia Following Anticancer Chemotherapy: A Population-Based Utilization Study in the Lazio Region. BioDrugs. 2017;31:117–124. doi: 10.1007/s40259-017-0214-9. - DOI - PMC - PubMed
-
- Kuderer N.M., Dale D.C., Crawford J., Lyman G.H. Impact of Primary Prophylaxis With Granulocyte Colony-Stimulating Factor on Febrile Neutropenia and Mortality in Adult Cancer Patients Receiving Chemotherapy: A Systematic Review. J. Clin. Oncol. 2007;25:3158–3167. doi: 10.1200/JCO.2006.08.8823. - DOI - PubMed
-
- Fan Z., Li Y., Zhao Q., Fan L., Tan B., Zuo J., Hua K., Ji Q. Highly Expressed Granulocyte Colony-Stimulating Factor (G-CSF) and Granulocyte Colony-Stimulating Factor Receptor (G-CSFR) in Human Gastric Cancer Leads to Poor Survival. Med. Sci. Monit. 2018;24:1701–1711. doi: 10.12659/MSM.909128. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

