Evaluation of Ultra-High Pressure Homogenization Treatments to Ensure the Microbiological Safety and Immunoglobulin Preservation in Donor Human Milk
- PMID: 40282712
- PMCID: PMC12025971
- DOI: 10.3390/foods14081310
Evaluation of Ultra-High Pressure Homogenization Treatments to Ensure the Microbiological Safety and Immunoglobulin Preservation in Donor Human Milk
Abstract
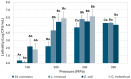
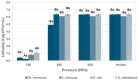
Most donor human milk (HM) banks use Holder pasteurization (HoP) to ensure microbiological safety, although it can degrade essential bioactive factors for newborns. This study evaluates the innovative ultra-high-pressure homogenization (UHPH) technology as a potential alternative. Listeria innocua, Staphylococcus carnosus, Franconibacter helveticus (formerly named Cronobacter helveticus) and Escherichia coli strains were used as surrogates for common HM pathogens according to European Milk Bank Association (EMBA) guidelines, to evaluate the efficacy of new technologies. A reconstituted powder milk formula inoculated with these strains was used to determine the most efficient conditions (those to achieve a lethality of ≥5 Log), applying treatments from 150 to 300 MPa. These treatments were later validated using inoculated HM with the same strains. Immunoglobulin (sIgA, IgG, IgM) retention was also evaluated and compared with HoP. Results showed that UHPH treatments at 200 MPa achieved a lethality > 5 Log for all strains, except for St. carnosus, which required 250 MPa for complete inactivation in HM. Unlike HoP, UHPH at 200 and 250 MPa did not significantly reduce the basal concentration of sIgA, IgG, or IgM compared with raw HM. These findings suggest UHPH as a promising alternative to HoP, maintaining both microbiological safety and immunological quality.
Keywords: UHPH; bioactive components; holder pasteurization; human milk; immunoglobulins; pathogen inactivation.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
High-pressure homogenization and high hydrostatic pressure processing of human milk: Preservation of immunological components for human milk banks.J Dairy Sci. 2020 Jul;103(7):5978-5991. doi: 10.3168/jds.2019-17569. Epub 2020 May 14. J Dairy Sci. 2020. PMID: 32418693
-
High-Pressure Processing of Human Milk: A Balance between Microbial Inactivation and Bioactive Protein Preservation.J Nutr. 2023 Sep;153(9):2598-2611. doi: 10.1016/j.tjnut.2023.07.001. Epub 2023 Jul 8. J Nutr. 2023. PMID: 37423385 Free PMC article.
-
Processing of Donor Human Milk: Update and Recommendations From the European Milk Bank Association (EMBA).Front Pediatr. 2019 Feb 28;7:49. doi: 10.3389/fped.2019.00049. eCollection 2019. Front Pediatr. 2019. PMID: 30873395 Free PMC article.
-
New alternatives to holder pasteurization in processing donor milk in human milk banks.Front Nutr. 2024 Jun 26;11:1409381. doi: 10.3389/fnut.2024.1409381. eCollection 2024. Front Nutr. 2024. PMID: 38988859 Free PMC article. Review.
-
The Effect of Holder Pasteurization on Nutrients and Biologically-Active Components in Donor Human Milk: A Review.Nutrients. 2016 Aug 2;8(8):477. doi: 10.3390/nu8080477. Nutrients. 2016. PMID: 27490567 Free PMC article. Review.
References
-
- Jarzynka S., Strom K., Barbarska O., Pawlikowska E., Minkiewicz-Zochniak A., Rosiak E., Oledzka G., Wesolowska A. Combination of High-Pressure Processing and Freeze-Drying as the Most Effective Techniques in Maintaining Biological Values and Microbiological Safety of Donor Milk. Int. J. Env. Res. Public Health. 2021;18:2147. doi: 10.3390/ijerph18042147. - DOI - PMC - PubMed
-
- Calvo J., García Lara N.R., Gormaz M., Peña M., Martínez Lorenzo M.J., Ortiz Murillo P., Brull Sabaté J.M., Samaniego C.M., Gayà A. Recomendaciones Para La Creación y El Funcionamiento de Los Bancos de Leche Materna En España. An. Pediatría. 2018;89:65.e1–65.e6. doi: 10.1016/j.anpedi.2018.01.010. - DOI - PubMed
-
- European Parliament and Council Regulation (EU) 2024/1938 of the European Parliament and of the Council of 13 June 2024 on Standards of Quality and Safety for Substances of Human Origin Intended for Human Application and Repealing Directives 2002/98/EC and 2004/23/EC. Off. J. Eur. Union. 2024;L series:80.
-
- Escuder-Vieco D., Rodríguez J.M., Espinosa-Martos I., Corzo N., Montilla A., García-Serrano A., Calvo M.V., Fontecha J., Serrano J., Fernández L., et al. High-Temperature Short-Time and Holder Pasteurization of Donor Milk: Impact on Milk Composition. Life. 2021;11:114. doi: 10.3390/life11020114. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

