Comparison of the Safety and Efficacy of Remimazolam and Propofol for Sedation in Adults Undergoing Colonoscopy: A Meta-Analysis of Randomized Controlled Trials
- PMID: 40282937
- PMCID: PMC12028776
- DOI: 10.3390/medicina61040646
Comparison of the Safety and Efficacy of Remimazolam and Propofol for Sedation in Adults Undergoing Colonoscopy: A Meta-Analysis of Randomized Controlled Trials
Abstract
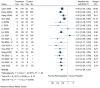
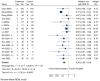
Background and Objectives: This meta-analysis evaluates the safety and efficacy of remimazolam versus propofol for sedation during colonoscopy, focusing on hemodynamic and respiratory outcomes. Materials and Methods: A comprehensive search of CENTRAL, Embase, PubMed, Scopus, and Web of Science up to January 2025 identified randomized controlled trials (RCTs). Outcomes included hypotension (primary outcome), bradycardia, respiratory depression, injection pain, sedation onset time, emergence time, procedure success rate, and recovery room stay. Effect sizes were reported as relative risks (RR) or mean differences (MD) using random-effects models. Results: Fourteen RCTs with 3290 participants were included. Remimazolam significantly reduced the risk of hypotension (RR: 0.44, 95% CI [0.39, 0.51], p = 0.0000), bradycardia (RR: 0.36, 95% CI [0.25, 0.53], p = 0.0000), respiratory depression (RR: 0.32, 95% CI [0.22, 0.45], p = 0.0000), and injection pain (RR: 0.14, 95% CI [0.09, 0.24], p = 0.0000) compared to propofol. Remimazolam had slower sedation onset (MD: 15.97 s, 95% CI [8.30, 23.64], p = 0.0000) but allowed faster emergence (MD: -0.91 min, 95% CI [-1.69, -0.13], p = 0.023) and shorter recovery room stays (MD: -2.20 min, 95% CI [-3.23, -1.17], p = 0.0000). Both drugs had similar procedure success rates. Conclusions: Remimazolam demonstrates superior safety and efficacy compared to propofol, reducing risks of hypotension, bradycardia, respiratory depression, and injection pain while enabling faster recovery. These findings support remimazolam as a viable sedative for colonoscopy, though further large-scale studies are needed to confirm these results.
Keywords: colonoscopy; hemodynamics; propofol; remimazolam; respiratory insufficiency; sedation.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
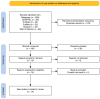

References
-
- Quaye A.N., Hisey W.M., Mackenzie T.A., Robinson C.M., Richard J.M., Anderson J.C., Warters R.D., Butterly L.F. Association Between Colonoscopy Sedation Type and Polyp Detection: A Registry-Based Cohort Study. Anesthesiology. 2024;140:1088–1097. doi: 10.1097/ALN.0000000000004955. - DOI - PMC - PubMed
-
- de Wit F., van Vliet A.L., de Wilde R.B., Jansen J.R., Vuyk J., Aarts L.P., de Jonge E., Veelo D.P., Geerts B.F. The effect of propofol on haemodynamics: Cardiac output, venous return, mean systemic filling pressure, and vascular resistances. Br. J. Anaesth. 2016;116:784–789. doi: 10.1093/bja/aew126. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

