Expression of 9- O-Acetylated Sialic Acid in HPV+ Oral Squamous Cell Carcinoma Cells
- PMID: 40283217
- PMCID: PMC12028955
- DOI: 10.3390/life15040663
Expression of 9- O-Acetylated Sialic Acid in HPV+ Oral Squamous Cell Carcinoma Cells
Abstract
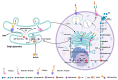
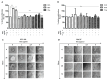
Oral squamous cell carcinoma (OSCC) is a common type of head and neck malignancy that represents a significant global health issue. Sialylations are common events in tumor transformation, proliferation, metastasis, and immune evasion. Modifications in sialylation can be detected by lectins, whose changes in OSCC have been related to grade, invasion, and metastasis. The presence of 9-O-acetylated sialic acid (Neu5,9Ac2) in OSCC cells and its potential expression, modification, and role are unknown. This study aimed to analyze the expression of Neu5,9Ac2 using the Macrobrachium rosenbergii lectin (MrL) that recognizes this sialic acid (Neu5Ac) residue and also compare its effect on the SCC-152 cell line (CRL-3240, ATCC) and immortalized keratinocytes (HaCaT) as a control. We observed by immunocytochemistry that SCC-152 cells expressed more Neu5,9Ac2 compared to HaCaT cells; the specificity of MrL was confirmed after the sialidase treatment of cells in which the loss of lectin's recognition of Neu5,9Ac2 was observed. The electrophoretic profile was similar between both cell line types; however, the Western blot showed differences in the glycoprotein patterns recognized by lectin for each cell type. MrL increased the proliferation of SCC-152 cells, as well as the integrity and morphology of the colonies. Therefore, our results suggest that Neu5,9Ac2 glycosylated receptors could be involved in the survival and proliferation of OSCC cells, which offers a promising avenue for developing diagnostic and prognostic tools (tumor markers) against oral squamous cell carcinoma in the future.
Keywords: HaCaT; Macrobrachium rosenbergii lectin; Neu5,9Ac2; Neu5Ac; OSCC; SCC-152; glycosylation; prognostic tools.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Fatima J., Fatima E., Mehmood F., Ishtiaq I., Khan M.A., Khurshid H.M.S., Kashif M. Comprehensive Analysis of Oral Squamous Cell Carcinomas: Clinical, Epidemiological, and Histopathological Insights with a Focus on Prognostic Factors and Survival Time. Cureus. 2024;16:e54394. doi: 10.7759/cureus.54394. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

