Recent Advances in Microfluidics-Based Monitoring of Waterborne Pathogens: From Isolation to Detection
- PMID: 40283337
- PMCID: PMC12029729
- DOI: 10.3390/mi16040462
Recent Advances in Microfluidics-Based Monitoring of Waterborne Pathogens: From Isolation to Detection
Abstract
Waterborne pathogens seriously threaten human life and can cause diarrhea, gastrointestinal disorders, and more serious systemic infections. These pathogens are usually caused by contaminated water sources that contain disease-causing microorganisms, such as bacteria, viruses, and parasites, which cause infection and disease when they enter the human body through drinking water or other means. Due to the wide range of transmission routes and the high potential risk of waterborne pathogens, there is an urgent need for an ultrasensitive, rapid, and specific pathogenic microorganism monitoring platform to meet the critical monitoring needs of some water bodies' collection points daily monitoring needs. Microfluidics-based pathogen surveillance methods are an important stage towards automated detection through real-time and multi-targeted monitoring, thus enabling a comprehensive assessment of the risk of exposure to waterborne pathogens and even emerging microbial contaminants, and thus better protection of public health. Therefore, this paper reviews the latest research results on the isolation and detection of waterborne pathogens based on microfluidic methods. First, we introduce the traditional methods for isolation and detection of pathogens. Then, we compare some existing microfluidic pathogen isolation and detection methods and finally look forward to some future research directions and applications of microfluidic technology in waterborne pathogens monitoring.
Keywords: detection; isolation; lab-on-a-chip (LOC); microfluidic chip; nucleic acid analysis; sample processing; waterborne pathogens.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



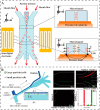

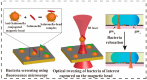



References
-
- Shukhratovich Abdullaev S., H Althomali R., Raza Khan A., Sanaan Jabbar H., Abosoda M., Ihsan A., Aggarwal S., Mustafa Y.F., Hammoud Khlewee I., Jabbar A.M. Integrating of Analytical Techniques with Enzyme-Mimicking Nanomaterials for the Fabrication of Microfluidic Systems for Biomedical Analysis. Talanta. 2024;273:125896. doi: 10.1016/j.talanta.2024.125896. - DOI - PubMed
Publication types
Grants and funding
- No. 2023YFA0915200,2023YFA0915204/This work was supported by grants from the National Key Research and Development Program of China
- PTYQ2024YZ0010/the equipment research and development projects of the ChineseAcademy of Sciences
- XTCX-KJ-2024-038/the Science and Technology Commission of Shanghai Municipality Project
- 22S31901700/the Science and Technology Commission of Shanghai Municipality
LinkOut - more resources
Full Text Sources
Miscellaneous

