Seeking Solutions for Inclusively Economic, Rapid, and Safe Molecular Detection of Respiratory Infectious Diseases: Comprehensive Review from Polymerase Chain Reaction Techniques to Amplification-Free Biosensing
- PMID: 40283347
- PMCID: PMC12029528
- DOI: 10.3390/mi16040472
Seeking Solutions for Inclusively Economic, Rapid, and Safe Molecular Detection of Respiratory Infectious Diseases: Comprehensive Review from Polymerase Chain Reaction Techniques to Amplification-Free Biosensing
Abstract
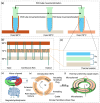
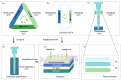
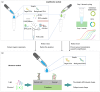
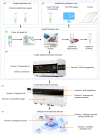
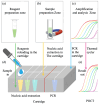
Frequent outbreaks of respiratory infectious diseases, driven by diverse pathogens, have long posed significant threats to public health, economic productivity, and societal stability. Respiratory infectious diseases are highly contagious, characterized by short incubation periods, diverse symptoms, multiple transmission routes, susceptibility to mutations, and distinct seasonality, contributing to their propensity for outbreaks. The absence of effective antiviral treatments and the heightened vulnerability of individuals with weakened immune systems make them more susceptible to infection, with severe cases potentially leading to complications or death. This situation becomes particularly concerning during peak seasons, such as influenza outbreaks. Therefore, early detection, diagnosis, and treatment are critical, alongside the prevention of cross-infection, ensuring patient safety, and controlling healthcare costs. To address these challenges, this review aims to identify a comprehensive, rapid, safe, and cost-effective diagnostic approach for respiratory infectious diseases. This approach is framed within the existing hierarchical healthcare system, focusing on establishing diagnostic capabilities at hospitals, community, and home levels to effectively tackle the above issues. In addition to PCR and isothermal amplification, the review also explores emerging molecular diagnostic strategies that may better address the evolving needs of respiratory disease diagnostics. A key focus is the transition from amplification technologies to amplification-free biosensing approaches, with particular attention given to their potential for home-based testing. This shift seeks to overcome the limitations of conventional amplification methods, particularly in decentralized and home diagnostics, offering a promising solution to enhance diagnostic speed and safety during outbreaks. In the future, with the integration of AI technologies into molecular amplification technologies, biosensors, and various application levels, the inclusively economic, rapid, and safe respiratory disease diagnosis solutions will be further optimized, and their accessibility will become more widespread.
Keywords: amplification-free biosensing; full scene solution; isothermal amplification; molecular detection of respiratory infectious diseases; polymerase chain reaction; tiered diagnosis.
Conflict of interest statement
Author Yaping Xie was employed by Sansure Biotech Inc. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
-
- COVID-19 Cases | WHO COVID-19 Dashboard. [(accessed on 10 March 2025)]. Available online: https://data.who.int/dashboards/covid19/cases?n=c.
-
- Hauck K. Oxford Research Encyclopedia of Economics and Finance. Oxford University Press; Oxford, UK: 2018. The Economics of Infectious Diseases.
Publication types
LinkOut - more resources
Full Text Sources

